Validation of a Clinical Prediction Rule for the Differentiation Between Septic Arthritis and Transient Synovitis of the Hip in Children by MININDER S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Knee Joint Surgery: Open Synovectomy
Musculoskeletal Surgical Services: Open Surgical Procedures; Knee Joint Surgery: Open Synovectomy POLICY INITIATED: 06/30/2019 MOST RECENT REVIEW: 06/30/2019 POLICY # HH-5588 Overview Statement The purpose of these clinical guidelines is to assist healthcare professionals in selecting the medical service that may be appropriate and supported by evidence to improve patient outcomes. These clinical guidelines neither preempt clinical judgment of trained professionals nor advise anyone on how to practice medicine. The healthcare professionals are responsible for all clinical decisions based on their assessment. These clinical guidelines do not provide authorization, certification, explanation of benefits, or guarantee of payment, nor do they substitute for, or constitute, medical advice. Federal and State law, as well as member benefit contract language, including definitions and specific contract provisions/exclusions, take precedence over clinical guidelines and must be considered first when determining eligibility for coverage. All final determinations on coverage and payment are the responsibility of the health plan. Nothing contained within this document can be interpreted to mean otherwise. Medical information is constantly evolving, and HealthHelp reserves the right to review and update these clinical guidelines periodically. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, or otherwise, without permission from HealthHelp. -

Ankle and Pantalar Arthrodesis
ANKLE AND PANTALAR ARTHRODESIS George E. Quill, Jr., M.D. In: Foot and Ankle Disorders Edited by Mark S. Myerson, M.D. Since reports in the late 19th Century, arthrodesis has been a successful accepted treatment method for painful disorders of the ankle, subtalar, and transverse tarsal joints. While the title of this chapter involves arthrodesis - the intentional fusion of a joint - as a form of reconstruction, this chapter will address not only surgical technique, but nonoperative methods of care as well. We will address the pathophysiology leading to ankle and hindfoot disability, succinctly review the existing literature on the topic of hindfoot and ankle arthrodesis, highlight the pathomechanics involved, and spend considerable time on establishing the diagnosis, indications, and preoperative planning when surgery is indicated. We also will discuss the rehabilitation of the postoperative patient, as well as the management of complications that may arise after ankle and pantalar arthrodesis. There are more than thirty different viable techniques that have been described in order to achieve successful ankle and hindfoot arthrodesis. It is not the purpose of this chapter to serve as compendium of all the techniques ever described. The author will, rather, attempt to distill into a useful amount of clinically applicable material this vast body of information that the literature and clinical experience provide. Ankle arthrodesis is defined as surgical fusion of the tibia to the talus. Surgical fusion of the ankle (tibiotalar) and subtalar (talocalcaneal) joints at the same operative sitting is termed tibiotalocalcaneal arthrodesis. Fusion of the talus to all the bones articulating with it (distal tibia, calcaneus, navicular, and cuboid) is termed pantalar arthrodesis. -

Knee Joint Surgery: Open Arthodesis of the Knee, Unspecified
Musculoskeletal Surgical Services: Open Surgical Procedures; Knee Joint Surgery: Open Arthodesis of the knee, unspecified POLICY INITIATED: 06/30/2019 MOST RECENT REVIEW: 06/30/2019 POLICY # HH-5623 Overview Statement The purpose of these clinical guidelines is to assist healthcare professionals in selecting the medical service that may be appropriate and supported by evidence to improve patient outcomes. These clinical guidelines neither preempt clinical judgment of trained professionals nor advise anyone on how to practice medicine. The healthcare professionals are responsible for all clinical decisions based on their assessment. These clinical guidelines do not provide authorization, certification, explanation of benefits, or guarantee of payment, nor do they substitute for, or constitute, medical advice. Federal and State law, as well as member benefit contract language, including definitions and specific contract provisions/exclusions, take precedence over clinical guidelines and must be considered first when determining eligibility for coverage. All final determinations on coverage and payment are the responsibility of the health plan. Nothing contained within this document can be interpreted to mean otherwise. Medical information is constantly evolving, and HealthHelp reserves the right to review and update these clinical guidelines periodically. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, or otherwise, without permission -

DISSERTATION INVESTIGATION of CATIONIC CONTRAST-ENHANCED COMPUTED TOMOGRAPHY for the EVALUATION of EQUINE ARTICULAR CARTILAGE Su
DISSERTATION INVESTIGATION OF CATIONIC CONTRAST-ENHANCED COMPUTED TOMOGRAPHY FOR THE EVALUATION OF EQUINE ARTICULAR CARTILAGE Submitted by Bradley B. Nelson Department of Clinical Sciences In partial fulfillment of the requirements For the Degree of Doctor of Philosophy Colorado State University Fort Collins, Colorado Fall 2017 Doctoral Committee: Advisor: Christopher E. Kawcak Co-Advisor: Laurie R. Goodrich C. Wayne McIlwraith Mark W. Grinstaff Myra F. Barrett Copyright by Bradley Bernard Nelson 2017 All Rights Reserved ABSTRACT INVESTIGATION OF CATIONIC CONTRAST-ENHANCED COMPUTED TOMOGRAPHY FOR THE EVALUATION OF EQUINE ARTICULAR CARTILAGE Osteoarthritis and articular cartilage injury are substantial problems in horses causing joint pain, lameness and decreased athleticism resonant of the afflictions that occur in humans. This debilitating joint disease causes progressive articular cartilage degeneration and coupled with a poor capacity to heal necessitates that articular cartilage injury is detected early before irreparable damage ensues. The use of diagnostic imaging is critical to identify and characterize articular cartilage injury, though currently available methods are unable to identify these early degenerative changes. Cationic contrast-enhanced computed tomography (CECT) uses a cationic contrast media (CA4+) to detect the early molecular changes that occur in the extracellular matrix. Glycosaminoglycans (GAGs) within the extracellular matrix are important for the providing the compressive stiffness of articular cartilage and their degradation is an early event in the development of osteoarthritis. Cationic CECT imaging capitalizes on the electrostatic attraction between CA4+ and GAGs; exposing the proportional relationship between the amount of GAGs present within and the amount of CA4+ that diffuses into the tissue. The amount of CA4+ that resides in the tissue is then quantified through CECT imaging and estimates tissue integrity through nondestructive assessment. -

Quantitative 3-Dimensional CT Analyses of Intramedullary Headless Screw Fixation for Metacarpal Neck Fractures
SCIENTIFIC ARTICLE Quantitative 3-Dimensional CT Analyses of Intramedullary Headless Screw Fixation for Metacarpal Neck Fractures Paul W. L. ten Berg, MSc, Chaitanya S. Mudgal, MD, Matthew I. Leibman, MD, Mark R. Belsky, MD, David E. Ruchelsman, MD Purpose Fixation countersunk beneath the articular surface is well accepted for periarticular fractures. Limited open intramedullary headless compression screw (HCS) fixation offers clinical advantages over Kirschner wire and open techniques. We used quantitative 3-di- mensional computed tomography to assess the articular starting point, surface area, and subchondral volumes used during HCS fixation of metacarpal neck fractures. Methods We simulated retrograde intramedullary insertion of 2.4- and 3.0-mm HCS and 1.1-mm Kirschner wires for metacarpal neck fracture fixation in 3-dimensional models from 16 adults. We used metacarpal head articular surface area (mm2) and subchondral volumes (mm3) and coronal and sagittal plane arcs of motion, during which we analyzed the center and rim of the articular base of the proximal phalanx engaging the countersunk entry site. Results Mean metacarpal head surface area mated to the proximal phalangeal base in neutral position was 93 mm2; through the coronal plane arc (45°), 129 mm2, and through the sagittal plane arc (120°), 265 mm2. The mean articular surface area used by countersunk HCS threads was 12%, 8%, and 4%, respectively, in each of these arcs. The 1.1-mm Kirschner wire occupied 1.2%, 0.9%, and 0.4%, respectively. Mean metacarpal head volume was 927 mm3. Mean subchondral volume occupied by the countersunk portion was 4%. The phalan- geal base did not overlap the dorsally located countersunk entry site through most of the sagittal plane arc. -

CMM-314: Hip Surgery-Arthroscopic and Open Procedures Version 1.0.2019
CLINICAL GUIDELINES CMM-314: Hip Surgery-Arthroscopic and Open Procedures Version 1.0.2019 Clinical guidelines for medical necessity review of speech therapy services. © 2019 eviCore healthcare. All rights reserved. Comprehensive Musculoskeletal Management Guidelines V1.0.2019 CMM-314: Hip Surgery-Arthroscopic and Open Procedures CMM-314.1: Definitions 3 CMM-314.2: General Guidelines 4 CMM-314.3: Indications and Non-Indications 4 CMM-314.4 Experimental, Investigational, or Unproven 6 CMM-314.5: Procedure (CPT®) Codes 7 CMM-314.6: References 10 © 2019 eviCore healthcare. All rights reserved. Page 2 of 13 400 Buckwalter Place Boulevard, Bluffton, SC 29910 • (800) 918-8924 www.eviCore.com Comprehensive Musculoskeletal Management Guidelines V1.0.2019 CMM-314.1: Definitions Femoroacetabular Impingement (FAI) is an anatomical mismatch between the head of the femur and the acetabulum resulting in compression of the labrum or articular cartilage during flexion. The mismatch can arise from subtle morphologic alterations in the anatomy or orientation of the ball-and-socket components (for example, a bony prominence at the head-neck junction or acetabular over-coverage) with articular cartilage damage initially occurring from abutment of the femoral neck against the acetabular rim, typically at the anterosui per or aspect of the acetabulum. Although hip joints can possess the morphologic features of FAI without symptoms, FAI may become pathologic with repetitive movement and/or increased force on the hip joint. High-demand activities may also result in pathologic impingement in hips with normal morphology. s It ha been proposed that impingement with damage to the labrum and/or acetabulum is a causative factor in the development of hip osteoarthritis, and that as many as half of cases currently categorized as primary osteoarthritis may have an etiology of FAI. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

Knee Replacement Surgery (Arthroplasty), Total and Partial
UnitedHealthcare® Commercial Medical Policy Surgery of the Knee Policy Number: 2021T0553S Effective Date: July 1, 2021 Instructions for Use Table of Contents Page Related Commercial Policy Coverage Rationale ........................................................................... 1 • Unicondylar Spacer Devices for Treatment of Pain Documentation Requirements......................................................... 1 or Disability Definitions ........................................................................................... 2 Community Plan Policy Applicable Codes .............................................................................. 2 • Surgery of the Knee U.S. Food and Drug Administration ................................................ 3 References ......................................................................................... 4 Medicare Advantage Coverage Summary Policy History/Revision Information................................................ 4 • Joints and Joint Procedures Instructions for Use ........................................................................... 5 Coverage Rationale Surgery of the Knee is proven and medically necessary in certain circumstances. For medical necessity clinical coverage criteria, refer to the InterQual® 2021, Apr. 2021 Release, CP: Procedures: • Arthroscopy, Diagnostic, +/- Synovial Biopsy, Knee • Arthroscopy or Arthroscopically Assisted Surgery, Knee • Arthrotomy, Knee • Total Joint Replacement (TJR), Knee • Removal and Replacement, Total Joint Replacement (TJR), -
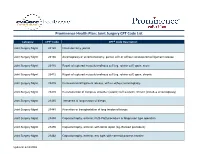
Master List of All CPT to Include Claims Only Codes for Any UM
Prominence Health Plan: Joint Surgery CPT Code List Category CPT® Code CPT® Code Description Joint Surgery Mgmt 23120 Claviculectomy; partial Joint Surgery Mgmt 23130 Acromioplasty or acromionectomy, partial, with or without coracoacromial ligament release Joint Surgery Mgmt 23410 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; acute Joint Surgery Mgmt 23412 Repair of ruptured musculotendinous cuff (eg, rotator cuff) open; chronic Joint Surgery Mgmt 23415 Coracoacromial ligament release, with or without acromioplasty Joint Surgery Mgmt 23420 Reconstruction of complete shoulder (rotator) cuff avulsion, chronic (includes acromioplasty) Joint Surgery Mgmt 23430 Tenodesis of long tendon of biceps Joint Surgery Mgmt 23440 Resection or transplantation of long tendon of biceps Joint Surgery Mgmt 23450 Capsulorrhaphy, anterior; Putti-Platt procedure or Magnuson type operation Joint Surgery Mgmt 23455 Capsulorrhaphy, anterior; with labral repair (eg, Bankart procedure) Joint Surgery Mgmt 23462 Capsulorrhaphy, anterior, any type; with coracoid process transfer Updated: 6/12/2018 Category CPT® Code CPT® Code Description Joint Surgery Mgmt 23466 Capsulorrhaphy, glenohumeral joint, any type multi-directional instability Joint Surgery Mgmt 23470 Arthroplasty, glenohumeral joint; hemiarthroplasty Arthroplasty, glenohumeral joint; total shoulder (glenoid and proximal humeral replacement (eg, Joint Surgery Mgmt 23472 total shoulder)) Revision of total shoulder arthroplasty, including allograft when performed; humeral or glenoid Joint -

Arthroscopy at AESC, We Are Proud to Offer Arthroscopy (Also Called
Arthroscopy At AESC, we are proud to offer arthroscopy (also called arthroscopic surgery), a new surgical procedure that allows us to evaluate and sometimes treat the inside of a joint in a minimally invasive fashion. It is a surgical procedure that uses a tiny viewing instrument called an ‘arthroscope’ that acts as a camera in conjunction with arthroscopic instruments (if treatment is needed). The surgical instruments used are much smaller than traditional instruments and instead of looking at the joint directly we view the joint area on a video monitor. During arthroscopy only two small incisions are made - one for the arthroscope and one for the surgical instrument(s). This minimally invasive surgery has a multitude of advantages over the conventional joint surgery of ‘arthrotomy’ (which means to fully open up the joint by an open approach through the muscles and an incision through the joint capsule). Some of those advantages include: • Due to the smaller size of the incisions, arthroscopy is less painful for the patient and allows for quicker recovery. • Due to the magnification provided with the camera, assessment of the joint is more complete and accurate. • The smaller approach causes less tissue trauma and therefore produces less scar tissue while the tissues are healing. • Due to the smaller approach, some of the complications associated with an open procedure (such as patella luxation after knee arthrotomy) can be avoided. Arthroscopy can be used to diagnose and sometimes also treat a joint problem. At AESC we commonly use arthroscopy for: • Confirmation of a tear of the cranial cruciate ligament in the knee • Treatment of meniscal injuries in the knee • Treatment of fragmentation of the coronoid process in the elbow (FCP) • Removal of cartilage flaps (OCD-lesions) from the shoulder, hock, knee and elbow • Assessment of the hip joint to evaluate the animal as a candidate for a Triple Pelvic Osteotomy (TPO) Complications from arthroscopy are rare. -

Hip Joint Surgery: Arthrotomy for Biopsy of the Sacroiliac Joint Or Hip Joint
Musculoskeletal Surgical Services: Hip Joint Surgery: Arthrotomy for biopsy of the sacroiliac joint or hip joint POLICY INITIATED: 06/30/2019 MOST RECENT REVIEW: 06/30/2019 POLICY # HH-5629 Overview Statement The purpose of these clinical guidelines is to assist healthcare professionals in selecting the medical service that may be appropriate and supported by evidence to improve patient outcomes. These clinical guidelines neither preempt clinical judgment of trained professionals nor advise anyone on how to practice medicine. The healthcare professionals are responsible for all clinical decisions based on their assessment. These clinical guidelines do not provide authorization, certification, explanation of benefits, or guarantee of payment, nor do they substitute for, or constitute, medical advice. Federal and State law, as well as member benefit contract language, including definitions and specific contract provisions/exclusions, take precedence over clinical guidelines and must be considered first when determining eligibility for coverage. All final determinations on coverage and payment are the responsibility of the health plan. Nothing contained within this document can be interpreted to mean otherwise. Medical information is constantly evolving, and HealthHelp reserves the right to review and update these clinical guidelines periodically. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, or otherwise, without permission from -

American Board of Foot and Ankle Surgery Logging Guidelines Revisions ABFAS Task Force
American Board of Foot and Ankle Surgery Logging Guidelines Revisions ABFAS Task Force Mindy Benton, DPM Randall Dei, DPM Charles Lombardi, DPM John Thomas Marcoux, DPM Roya Mirmiran, DPM Michael Vaardahl, DPM Appendix B: Surgical Procedure Categories and Code Numbers 1. Digital Surgery (lesser toe or hallux) 1.1 partial ostectomy/exostectomy 1.2 phalangectomy 1.3 arthroplasty (interphalangeal joint [IPJ]) 1.4 implant (IPJ) (silastic implant or spacer) 1.5 diaphysectomy 1.6 phalangeal osteotomy 1.7 fusion (IPJ) 1.8 amputation 1.9 management of osseous tumor/neoplasm 1.10 management of bone/joint infection 1.11 open management of digital fracture/dislocation 1.12 revision/repair of surgical outcome 1.13 other osseous digital procedure not listed above Appendix B: Surgical Procedure Categories and Code Numbers 2. First Ray Surgery Hallux Valgus Surgery 2.1.1 bunionectomy (partial ostectomy/Silver procedure), with or without capsulotendon balancing procedure 2.1.2(procedure code number no longer used) 2.1.3 bunionectomy with phalangeal osteotomy 2.1.4 bunionectomy with distal first metatarsal osteotomy 2.1.5 bunionectomy with first metatarsal base or shaft osteotomy 2.1.6 bunionectomy with first metatarsocuneiform fusion 2.1.7 metatarsophalangeal joint (MPJ) fusion 2.1.8 MPJ implant 2.1.9 MPJ arthroplasty 2.1.10 bunionectomy double correction with osteotomy and/or arthrodesis Appendix B: Surgical Procedure Categories and Code Numbers • Hallux Limitus Surgery 2.2.1 cheilectomy 2.2.2 joint salvage with phalangeal osteotomy (Kessel-Bonney, enclavement)