Developmental Changes in Story-Evoked Responses in the Neocortex and Hippocampus Samantha S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Prefrontal Cortex of the Primate: a Synopsis
Psychobiology 2000,28 (2),125-13/ The prefrontal cortex of the primate: A synopsis JOAQuiN M. FUSTER University of California, Los Angeles, California The prefrontal cortex is one of the latest regions of the neocortex to develop, in both phylogeny and ontogeny. In the primate, the prefrontal cortex is anatomically divided into three major sectors: medial, orbital (or inferior), and dorsolateral. The dorsolateral sector is the association cortex of the convex ity of the frontal lobe. Phylogenetically and ontogenetically, this part of the prefrontal cortex is the one to develop last and most. It is the neural substrate of the higher cognitive functions that reach their maximum development in the human brain. The-most general and distinctive function of the dorso lateral prefrontal cortex is the temporal organization of goal-directed actions. In the human, this role extends to the domains of speech and reasoning. Two temporally symmetrical and mutually comple mentary cognitive functions-one retrospective and the other prospective-support that general pre frontal function of temporal organization: (1) active short-term memory, also called working memory; (2) prospective or preparatory set. The dorsolateral prefrontal cortex interacts with other cortical and subcortical structures in those two time-bridging functions at the basis of the temporal organization of behavior. This article presents in summary form the most salient the cortex of the dorsal and lateral convexity ofthe ante empirical facts known to date on the structure and func rior part of the frontal lobe (Brodmann's cytoarchitectonic tions of the prefrontal cortex of the human and nonhuman area 46, and lateral parts of areas 8, 9, 10, and 11); (2) me primate. -

Corticostriatal Interactions During Learning, Memory Processing, and Decision Making
The Journal of Neuroscience, October 14, 2009 • 29(41):12831–12838 • 12831 Mini-Symposium Corticostriatal Interactions during Learning, Memory Processing, and Decision Making Cyriel M. A. Pennartz,1 Joshua D. Berke,2,3 Ann M. Graybiel,4,5 Rutsuko Ito,6 Carien S. Lansink,1 Matthijs van der Meer,7 A. David Redish,7 Kyle S. Smith,4,5 and Pieter Voorn8 1University of Amsterdam, Swammerdam Institute for Life Sciences Center for Neuroscience, 1098 XH Amsterdam, The Netherlands, 2Department of Psychology and 3Neuroscience Program, University of Michigan, Ann Arbor, Michigan 48109, 4Department of Brain and Cognitive Sciences and 5McGovern Institute for Brain Research, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, 6Department of Experimental Psychology, University of Oxford, Oxford OX1 3UD, United Kingdom, 7Department of Neuroscience, University of Minnesota, Minneapolis, Minnesota 55455, and 8Department of Anatomy, Research Institute Neurosciences, Vrije Universiteit University Medical Center, 1007 MB Amsterdam, The Netherlands This mini-symposium aims to integrate recent insights from anatomy, behavior, and neurophysiology, highlighting the anatom- ical organization, behavioral significance, and information-processing mechanisms of corticostriatal interactions. In this sum- mary of topics, which is not meant to provide a comprehensive survey, we will first review the anatomy of corticostriatal circuits, comparing different ways by which “loops” of cortical–basal ganglia circuits communicate. Next, we will address the causal importance and systems-neurophysiological mechanisms of corticostriatal interactions for memory, emphasizing the communi- cation between hippocampus and ventral striatum during contextual conditioning. Furthermore, ensemble recording techniques have been applied to compare information processing in the dorsal and ventral striatum to predictions from reinforcement learning theory. -

Cortical Layers: What Are They Good For? Neocortex
Cortical Layers: What are they good for? Neocortex L1 L2 L3 network input L4 computations L5 L6 Brodmann Map of Cortical Areas lateral medial 44 areas, numbered in order of samples taken from monkey brain Brodmann, 1908. Primary visual cortex lamination across species Balaram & Kaas 2014 Front Neuroanat Cortical lamination: not always a six-layered structure (archicortex) e.g. Piriform, entorhinal Larriva-Sahd 2010 Front Neuroanat Other layered structures in the brain Cerebellum Retina Complexity of connectivity in a cortical column • Paired-recordings and anatomical reconstructions generate statistics of cortical connectivity Lefort et al. 2009 Information flow in neocortical microcircuits Simplified version “computational Layer 2/3 layer” Layer 4 main output Layer 5 main input Layer 6 Thalamus e - excitatory, i - inhibitory Grillner et al TINS 2005 The canonical cortical circuit MAYBE …. DaCosta & Martin, 2010 Excitatory cell types across layers (rat S1 cortex) Canonical models do not capture the diversity of excitatory cell classes Oberlaender et al., Cereb Cortex. Oct 2012; 22(10): 2375–2391. Coding strategies of different cortical layers Sakata & Harris, Neuron 2010 Canonical models do not capture the diversity of firing rates and selectivities Why is the cortex layered? Do different layers have distinct functions? Is this the right question? Alternative view: • When thinking about layers, we should really be thinking about cell classes • A cells class may be defined by its input connectome and output projectome (and some other properties) • The job of different classes is to (i) make associations between different types of information available in each cortical column and/or (ii) route/gate different streams of information • Layers are convenient way of organising inputs and outputs of distinct cell classes Excitatory cell types across layers (rat S1 cortex) INTRATELENCEPHALIC (IT) | PYRAMIDAL TRACT (PT) | CORTICOTHALAMIC (CT) From Cereb Cortex. -

Neocortex: Consciousness Cerebellum
Grey matter (chips) White matter (the wiring: the brain mainly talks to itself) Neocortex: consciousness Cerebellum: unconscious control of posture & movement brains 1. Golgi-stained section of cerebral cortex 2. One of Ramon y Cajal’s faithful drawings showing nerve cell diversity in the brain cajal Neuropil: perhaps 1 km2 of plasma membrane - a molecular reaction substrate for 1024 voltage- and ligand-gated ion channels. light to Glia: 3 further cell types 1. Astrocytes: trophic interface with blood, maintain blood brain barrier, buffer excitotoxic neurotransmitters, support synapses astros Oligodendrocytes: myelin insulation oligos Production persists into adulthood: radiation myelopathy 3. Microglia: resident macrophages of the CNS. Similarities and differences with Langerhans cells, the professional antigen-presenting cells of the skin. 3% of all cells, normally renewed very slowly by division and immigration. Normal Neurosyphilis microglia Most adult neurons are already produced by birth Peak synaptic density by 3 months EMBRYONIC POSTNATAL week: 0 6 12 18 24 30 36 Month: 0 6 12 18 24 30 36 Year: 4 8 12 16 20 24 Cell birth Migration 2* Neurite outgrowth Synaptogenesis Myelination 1* Synapse elimination Modified from various sources inc: Andersen SL Neurosci & Biobehav Rev 2003 Rakic P Nat Rev Neurosci 2002 Bourgeois Acta Pediatr Suppl 422 1997 timeline 1 Synaptogenesis 100% * Rat RTH D BI E A Density of synapses in T PUBERTY primary visual cortex H at different times post- 0% conception. 100% (logarithmic scale) RTH Cat BI D E A T PUBERTY H The density values equivalent 0% to 100% vary between species 100% but in Man the peak value is Macaque 6 3 RTH 350 x10 synapses per mm BI D E PUBERTY A T The peak rate of synapse H formation is at birth in the 0% macaque: extrapolating to 100% the entire cortex, this Man RTH BI amounts to around 800,000 D E synapses formed per sec. -

The Neocortex of Cetartiodactyls. II. Neuronal Morphology of the Visual and Motor Cortices in the Giraffe (Giraffa Camelopardalis)
Brain Struct Funct (2015) 220:2851–2872 DOI 10.1007/s00429-014-0830-9 ORIGINAL ARTICLE The neocortex of cetartiodactyls. II. Neuronal morphology of the visual and motor cortices in the giraffe (Giraffa camelopardalis) Bob Jacobs • Tessa Harland • Deborah Kennedy • Matthew Schall • Bridget Wicinski • Camilla Butti • Patrick R. Hof • Chet C. Sherwood • Paul R. Manger Received: 11 May 2014 / Accepted: 21 June 2014 / Published online: 22 July 2014 Ó Springer-Verlag Berlin Heidelberg 2014 Abstract The present quantitative study extends our of aspiny neurons in giraffes appeared to be similar to investigation of cetartiodactyls by exploring the neuronal that of other eutherian mammals. For cross-species morphology in the giraffe (Giraffa camelopardalis) neo- comparison of neuron morphology, giraffe pyramidal cortex. Here, we investigate giraffe primary visual and neurons were compared to those quantified with the same motor cortices from perfusion-fixed brains of three su- methodology in African elephants and some cetaceans badults stained with a modified rapid Golgi technique. (e.g., bottlenose dolphin, minke whale, humpback whale). Neurons (n = 244) were quantified on a computer-assis- Across species, the giraffe (and cetaceans) exhibited less ted microscopy system. Qualitatively, the giraffe neo- widely bifurcating apical dendrites compared to ele- cortex contained an array of complex spiny neurons that phants. Quantitative dendritic measures revealed that the included both ‘‘typical’’ pyramidal neuron morphology elephant and humpback whale had more extensive den- and ‘‘atypical’’ spiny neurons in terms of morphology drites than giraffes, whereas the minke whale and bot- and/or orientation. In general, the neocortex exhibited a tlenose dolphin had less extensive dendritic arbors. -

NEUROGENESIS in the ADULT BRAIN: New Strategies for Central Nervous System Diseases
7 Jan 2004 14:25 AR AR204-PA44-17.tex AR204-PA44-17.sgm LaTeX2e(2002/01/18) P1: GCE 10.1146/annurev.pharmtox.44.101802.121631 Annu. Rev. Pharmacol. Toxicol. 2004. 44:399–421 doi: 10.1146/annurev.pharmtox.44.101802.121631 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on August 28, 2003 NEUROGENESIS IN THE ADULT BRAIN: New Strategies for Central Nervous System Diseases ,1 ,2 D. Chichung Lie, Hongjun Song, Sophia A. Colamarino,1 Guo-li Ming,2 and Fred H. Gage1 1Laboratory of Genetics, The Salk Institute, La Jolla, California 92037; email: [email protected], [email protected], [email protected] 2Institute for Cell Engineering, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21287; email: [email protected], [email protected] Key Words adult neural stem cells, regeneration, recruitment, cell replacement, therapy ■ Abstract New cells are continuously generated from immature proliferating cells throughout adulthood in many organs, thereby contributing to the integrity of the tissue under physiological conditions and to repair following injury. In contrast, repair mechanisms in the adult central nervous system (CNS) have long been thought to be very limited. However, recent findings have clearly demonstrated that in restricted areas of the mammalian brain, new functional neurons are constantly generated from neural stem cells throughout life. Moreover, stem cells with the potential to give rise to new neurons reside in many different regions of the adult CNS. These findings raise the possibility that endogenous neural stem cells can be mobilized to replace dying neurons in neurodegenerative diseases. -

Rat Brains Also Have a Default Mode Network
Rat brains also have a default mode network Hanbing Lua, Qihong Zoua,b, Hong Gua, Marcus E. Raichlec,1, Elliot A. Steina,1, and Yihong Yanga,1 aNeuroimaging Research Branch, National Institute on Drug Abuse, Intramural Research Programs, National Institutes of Health, Baltimore, MD 21224; bMRI Research Center and Beijing City Key Laboratory for Medical Physics and Engineering, Peking University, Beijing 100871, China; and cDepartments of Radiology, Neurology, Anatomy and Neurobiology, and Biomedical Engineering, Washington University in St. Louis, St. Louis, MO 63110 Contributed by Marcus E. Raichle, January 11, 2012 (sent for review August 16, 2011) The default mode network (DMN) in humans has been suggested networks based on synchronized spontaneous fluctuations (12, to support a variety of cognitive functions and has been implicated 13). Because many anesthetic agents interfere with neural ac- in an array of neuropsychological disorders. However, its function tivity and neurovascular coupling, we first developed an anes- (s) remains poorly understood. We show that rats possess a DMN thesia regime consisting of a combination of dexmedetomidine that is broadly similar to the DMNs of nonhuman primates and and isoflurane. In a pilot study (n = 11), the functional status of humans. Our data suggest that, despite the distinct evolutionary the rat brain under the above anesthetic regime was assessed by paths between rodent and primate brain, a well-organized, intrin- measuring fMRI responses to electrical forepaw stimulation. sically coherent DMN appears to be a fundamental feature in the Each rat was studied on two occasions separated by 1 wk. As mammalian brain whose primary functions might be to integrate shown in Fig. -

Frontal Lobe and Cognitive Development
Journal of Neurocytology 31, 373–385 (2002) Frontal lobe and cognitive development JOAQUIN´ M. FUSTER Neuropsychiatric Institute and Brain Research Institute, UCLA School of Medicine Los Angeles, California [email protected] Received December 1, 2002; accepted December 12, 2002 Abstract In phylogeny as in ontogeny, the association cortex of the frontal lobe, also known as the prefrontal cortex, is a late-developing region of the neocortex. It is also one of the cortical regions to undergo the greatest expansion in the course of both evolution and individual maturation. In the human adult, the prefrontal cortex constitutes as much as nearly one-third of the totality of the neocortex. The protracted, relatively large, development of the prefrontal cortex is manifest in gross morphology as well as fine structure. In the developing individual, its late maturation is made most apparent by the late myelination of its axonal connections. This and other indices of morphological development of the prefrontal cortex correlate with the development of cognitive functions that neuropsychological studies in animals and humans have ascribed to this cortex. In broad outline, the ventromedial areas of the prefrontal cortex, which with respect to other prefrontal areas develop relatively early, are involved in the expression and control of emotional and instinctual behaviors. On the other hand, the late maturing areas of the lateral prefrontal convexity are principally involved in higher executive functions. The most general executive function of the lateral prefrontal cortex is the temporal organization of goal-directed actions in the domains of behavior, cognition, and language. In all three domains, that global function is supported by a fundamental role of the lateral prefrontal cortex in temporal integration, that is, the integration of temporally discontinuous percepts and neural inputs into coherent structures of action. -

Satb2 Is Required for the Regionalization of Retrosplenial Cortex
Cell Death & Differentiation (2020) 27:1604–1617 https://doi.org/10.1038/s41418-019-0443-1 ARTICLE Satb2 is required for the regionalization of retrosplenial cortex 1 2 1,2 1 1 3,4 1 Lei Zhang ● Ning-Ning Song ● Qiong Zhang ● Wan-Ying Mei ● Chun-Hui He ● Pengcheng Ma ● Ying Huang ● 1 3,4 1,5 1,2,6 Jia-Yin Chen ● Bingyu Mao ● Bing Lang ● Yu-Qiang Ding Received: 23 March 2019 / Revised: 10 October 2019 / Accepted: 11 October 2019 / Published online: 30 October 2019 © The Author(s) 2019. This article is published with open access Abstract The retrosplenial cortex (Rsp) is a transitional cortex located between the neocortex and archicortex, but the molecular mechanism specifying Rsp from the archicortex remains elusive. We here report that the transcription factor Satb2 is required for specifying Rsp identity during its morphogenesis. In Satb2 CKO mice, the boundary between the Rsp and archicortex [i.e., subiculum (SubC)] disappears as early as E17.5, and Rsp efferent projection is aberrant. Rsp-specific genes are lost, whereas SubC-specific genes are ectopically expressed in Rsp of Satb2 CKO mice. Furthermore, cell-autonomous role of Satb2 in maintaining Rsp neuron identity is revealed by inactivation of Satb2 in Rsp neurons. Finally, Satb2 represses the transcription of Nr4a2. The misexpression of Nr4a2 together with Ctip2 induces expression of SubC-specific genes in wild-type Rsp, and simultaneous knockdown of these two genes in Rsp Satb2-mutant cells prevents their fate transition to SubC identity. Thus, 1234567890();,: 1234567890();,: Satb2 serves as a determinant gene in the Rsp regionalization by repressing Nr4a2 and Ctip2 during cortical development. -

Time-Varying Covariance of Neural Activities Recorded in Striatum and Frontal Cortex As Monkeys Perform Sequential-Saccade Tasks
Time-varying covariance of neural activities recorded in striatum and frontal cortex as monkeys perform sequential-saccade tasks N. Fujii*† and A. M. Graybiel*‡ *Department of Brain and Cognitive Sciences and the McGovern Institute for Brain Research, Massachusetts Institute of Technology, 45 Carleton Street, E25-618, Cambridge, MA 02139; and †Laboratory for Symbolic Cognitive Development, Brain Science Institute, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan Contributed by A. M. Graybiel, May 2, 2005 Cortico-basal ganglia circuits are key parts of the brain’s habit circuit in macaque monkeys and simultaneously recorded neural system, but little is yet known about how these forebrain path- activity in these regions with multiple chronically implanted ways function as ingrained habits are performed. We simulta- electrodes as the monkeys viewed a sequence of visual targets neously recorded spike and local field potential (LFP) activity from and made saccades to each in order to receive reward. We regions of the frontal cortex and basal ganglia implicated in recorded from the oculomotor region of the caudate nucleus, the visuo-oculomotor control as highly trained macaque monkeys dorsolateral prefrontal cortex anterior to the frontal eye fields, performed sequences of visually guided saccades. The tasks were in which we have found saccade-related activity (10) and, in some repetitive, required no new learning, and could be performed experiments, the supplementary eye field (SEF) as well. To nearly automatically. Our findings demonstrate striking differ- achieve a population-level analysis, we recorded local field ences between the relative timing of striatal and cortical activity potential (LFP) activity (11–15) in addition to the spike activity during performance of the tasks. -
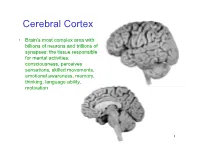
Cerebral Cortex
Cerebral Cortex • Brain’s most complex area with billions of neurons and trillions of synapses: the tissue responsible for mental activities: consciousness, perceives sensations, skilled movements, emotional awareness, memory, thinking, language ability, motivation 1 John Lorber, Science 210:1232 (1980) “There a young student at this university who has an IQ of 126, has gained a first-class honors degree in mathematics, and is socially completely normal. And yet the boy has virtually no brain.” “The student’s physician at the university noticed that the student had slightly larger than normal head… When we did a brain scan on him we saw that instead of the normal 4.5 cm thickness of brain tissue there was just a millimeter or so. His cranium is filled with CSF.” How is this possible? What does it tell us? Do you think this would be OK if it happened to an adult? To a 15 year old? To a 5 year old? To a neonate? 3 Types of Cerebral Cortex • Neocortex – Newest in evolution – About 90% of total – 6 layers, most complex • Paleocortex – Associated with olfactory system, the parahippocampal gyrus, uncus – fewer than 6 layers • Archicortex – Hippocampal formation; limbic system – 3 layers, most primitive • Mesocortex – Cingulate gyrus, insular cortex – Transitional between archicortex and neocortex 5 The perks of having a neocortex • The words used to describe the higher mental capacities of animals with a large neocortex include: – CONSCIOUSNESS – FREE WILL – INTELLIGENCE – INSIGHT • Animals with much simpler brains learn well, so LEARNING should not be among these capacities (Macphail 1982). • A species could have genetically determined mechanisms, acquired through evolutionary selection, for taking advantage of the regular features of the environment, or they could have learned through direct experience. -

Roles of Cajal-Retzius Cells Ioana Genescu
Assembling layer 1 of the neocortex : roles of Cajal-Retzius cells Ioana Genescu To cite this version: Ioana Genescu. Assembling layer 1 of the neocortex : roles of Cajal-Retzius cells. Neurons and Cognition [q-bio.NC]. Université Paris sciences et lettres, 2020. English. NNT : 2020UPSLE007. tel-03188109 HAL Id: tel-03188109 https://tel.archives-ouvertes.fr/tel-03188109 Submitted on 1 Apr 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Préparée à l’Institut de Biologie de l’École Normale Supérieure L'assemblage de la couche 1 du néocortex : Rôles des cellules de Cajal-Retzius Assembling layer 1 of the neocortex: Roles of Cajal-Retzius cells Soutenue par Composition du jury : Ioana GENESCU Patricia, GASPAR Le 08 octobre 2020 DR, Institut du Cerveau, Paris Présidente Alain, CHÉDOTAL DR, Institut de la Vision, Paris Rapporteur Ecole doctorale n° 515 Denis, JABAUDON Prof., Université de Genève, Genève Rapporteur Complexité du Vivant Alexandre, BAFFET CR., Institut Curie, Paris Examinateur Alessandra, PIERANI DR., Institut Imagine, IPNP, Paris Examinatrice Spécialité Sonia, GAREL Neurosciences Prof., IBENS, Collège de France, Paris Directrice de thèse Biologie du développement TABLE OF CONTENTS LIST OF ABBREVIATIONS 10 INTRODUCTION 14 Preablme 15 1.