Stable Isotope and Scat Analyses Indicate Diet and Habitat Partitioning in Northern Fur Seals Callorhinus Ursinus Across the Eastern Pacific
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Forage Fish Management Plan
Oregon Forage Fish Management Plan November 19, 2016 Oregon Department of Fish and Wildlife Marine Resources Program 2040 SE Marine Science Drive Newport, OR 97365 (541) 867-4741 http://www.dfw.state.or.us/MRP/ Oregon Department of Fish & Wildlife 1 Table of Contents Executive Summary ....................................................................................................................................... 4 Introduction .................................................................................................................................................. 6 Purpose and Need ..................................................................................................................................... 6 Federal action to protect Forage Fish (2016)............................................................................................ 7 The Oregon Marine Fisheries Management Plan Framework .................................................................. 7 Relationship to Other State Policies ......................................................................................................... 7 Public Process Developing this Plan .......................................................................................................... 8 How this Document is Organized .............................................................................................................. 8 A. Resource Analysis .................................................................................................................................... -

Fishery Bulletin/U S Dept of Commerce National Oceanic
Abstract.- Gastrointestinal tract contents were evaluated from Prey selection by northern fur seals 73 female andjuvenile male north ern fur seals (Callorhinus ursinus) fCallorhinus ursinusJ in the eastern for analysis of their diet in the Bering Sea. Fur seals were col Bering Sea lected from August to October of 1981, 1982, and 1985. Juvenile Elizabeth Sinclair walleye pollock (Theragra chalco gramma) and gonatid squid were Thomas Loughlin the primary prey. Pacific herring National Marine Mammal Laboratory. Alaska Fisheries Science Center; (Clupea pallasi) and capelin National Marine Fisheries Service. NOM <Mallotus villosus), considered im 7600 Sand Point Way. N.E., Seattle, Washington 98115 portant fur seal prey in previous reports, were absent from the diet. Prey species and size varied William Pearcy among years and between near College of Oceanography, Oregon State University shore and pelagic sample loca Oceanography Administration Building 104 tions. Interannual variation in the Corvaflis, Oregon 9733 J importance ofpollock in the diet of fur seals was positively related to year-class strength of pollock. Midwater (n=23) and bottom (n=116) trawls were conducted at The Pribilof Island population (St. were conducted prior to the 1975 the location of fur seal collections George and St. Paul Islands) of 81 population decline and prior to to determine availability of fish northern fur seals (Callorhinus the 1970's development of a com and squid relative to prey species eaten by fur seals. The species and ursinus) represents approximately mercial walleye pollock (Theragra size composition of prey taken by 75% of the total species breeding chalcogramma) fishery in the fur seals was similar to midwater population. -

Full Text in Pdf Format
MARINE ECOLOGY PROGRESS SERIES Vol. 177: 115-131, 1999 Published February 11 Mar Ecol Prog Ser Diet, provisioning and productivity responses of marine predators to differences in availability of Antarctic krill J. P. Croxall*, K. Reid, P. A. Prince British Antarctic Survey, Natural Environment Research Council. High Cross, Madingley Road. Cambridge CB3 OET. United Kingdom ABSTRACT: Knowledge of relationships between prey availability and predator performance is the key to using predators as indicators of the state of marine systems and to assessing potential conse- quences of competition between natural predators and man for common resources. Fluctuations in the abundance of Antarctic knll are beiieved to have a substantial influence on the reproductive perfor- mance of krill-dependent top predator species in the Southern Ocean; few quantifications of such inter- actions exist. At South Georgia, for 2 years in which acoustic surveys revealed a major difference in knll abundance, we compared diet, prov~sioningof offspring and breeding success in 4 main predator species (2 penguins, 2 albatrosses, with supporting data from Antarctic fur seal) whose dependence on krd typically ranges from 20 to 90%. The 4-fold difference in krill biomass between 1986 (ca 30 g m-2) and 1994 (ca 7 g m-2)was accompanied by (1) an 88 to 90% reduction in the mass of krill in predator dlets (and some increase in the fish component), (2) greater prey diversity for most species, (3) reduced diet overlap between species and (4) a switch from krill to amphipods in macaroni penguin but no major dietary change in other species. -

Long-Term Fluctuations in Gonatid Squid (Gonatidae) Abundance in the Okhotsk Sea
Pacific Research Institute of Fisheries and Oceanography (TINRO-Centre) Long-term fluctuations in gonatid squid (Gonatidae) abundance in the Okhotsk Sea Mikhail A. Zuev Nikolai S. Vanin Oleg N. Katugin Gennady A. Shevtsov The main goal reveal annual distribution patterns of squids of the family Gonatidae in the Sea of Okhotsk basing on data collected in TINRO-Centre research surveys with a purpose to estimate the role of these cephalopods in pelagic communities Methods: – analyze database on squid catches for a period from 1990 to 2008; – use the data to obtain patterns of spatial and seasonal on squid distribution and biology; – assess the role of different species in seasonal dynamics of distribution density in the epi- and mesopelagic layers in different years; – use synoptic observations to reveal relationships between squid distribution density and variability in atmospheric pressure in the study area Upper-mesopelagic Region expeditions investigations years (expeditions) northern Okhotsk Sea 20 12 1990-2008 southern 18 2 1990-2008 Okhotsk Sea off west 12 7 1998-2007 Kamchatka the entire Okhotsk Sea 25 13 1990-2008 - Investigations of epipelagic (0-200 m) - Investigations of epipelagic and upper- mesopelagic (200-500 m) northern Okhotsk Sea Kamchatka Biomass density (kg/km2) of the Gonatidae in the northern Okhotsk Sea kg/km2 0,6 2 - Berryteuthis magister 0,5 4000,4 - Gonatus madokai 0,3 2000,2 0,1 0 w w s s о w s s о w s s о w s s о w s s о о w s s о w s s s о 1991 ] [ 1993][1994 1995 ][ 1997 1998 1999 2000 1,2 - Boreoteuthis borealis -

An Isotope Approach
Determining Cetacean – Cephalopod Trophic Interactions – An Isotope Approach By Christine Heather Jackson Bachelor of Arts, James Cook University Graduate Diploma (Health Promotion), Curtin University of Technology Master of Science, University of Tasmania Submitted in fulfilment of the requirements for the degree Doctor of Philosophy University of Tasmania (October 2016) ii DECLARATION This thesis contains no material which has been accepted for a degree or diploma by the University or any other institution, except by way of background information and duly acknowledged in the thesis, and to the best of my knowledge and belief no material previously published or written by another person except where due acknowledgement is made in the text of the thesis, nor does the thesis contain any material that infringes copyright Signed; _________________________________ Christine H. Jackson iii AUTHORITY OF ACCESS This thesis may be made available for loan and limited copying and communication in accordance with the Copyright Act 1968. Signed; _________________________________ Christine H. Jackson iv ABSTRACT Determining the biotic and abiotic influences on the distribution and abundance of marine mammals is essential for understanding the dynamics of the food chain. The predator-prey relationship can be deterministic in shaping both the community structure and function of marine ecosystems. This is especially pertinent to recovering toothed whale populations given their large size and high prey consumption rates. A greater knowledge of the trophic linkages between toothed whales and their prey will facilitate assessments of their combined impact on the ecosystem since marine food webs are a fusion of bottom-up and top-down energy and nutrient flow. -

XIV. Appendices
Appendix 1, Page 1 XIV. Appendices Appendix 1. Vertebrate Species of Alaska1 * Threatened/Endangered Fishes Scientific Name Common Name Eptatretus deani black hagfish Lampetra tridentata Pacific lamprey Lampetra camtschatica Arctic lamprey Lampetra alaskense Alaskan brook lamprey Lampetra ayresii river lamprey Lampetra richardsoni western brook lamprey Hydrolagus colliei spotted ratfish Prionace glauca blue shark Apristurus brunneus brown cat shark Lamna ditropis salmon shark Carcharodon carcharias white shark Cetorhinus maximus basking shark Hexanchus griseus bluntnose sixgill shark Somniosus pacificus Pacific sleeper shark Squalus acanthias spiny dogfish Raja binoculata big skate Raja rhina longnose skate Bathyraja parmifera Alaska skate Bathyraja aleutica Aleutian skate Bathyraja interrupta sandpaper skate Bathyraja lindbergi Commander skate Bathyraja abyssicola deepsea skate Bathyraja maculata whiteblotched skate Bathyraja minispinosa whitebrow skate Bathyraja trachura roughtail skate Bathyraja taranetzi mud skate Bathyraja violacea Okhotsk skate Acipenser medirostris green sturgeon Acipenser transmontanus white sturgeon Polyacanthonotus challengeri longnose tapirfish Synaphobranchus affinis slope cutthroat eel Histiobranchus bathybius deepwater cutthroat eel Avocettina infans blackline snipe eel Nemichthys scolopaceus slender snipe eel Alosa sapidissima American shad Clupea pallasii Pacific herring 1 This appendix lists the vertebrate species of Alaska, but it does not include subspecies, even though some of those are featured in the CWCS. -

Redacted for Privacy William G
AN ABSTRACT OF THE THESIS OF Elizabeth H. Sinclair for the degree of Master of Science in Oceanography presented on December 16, 1988. Title: Feeding Habits of Northern Fur Seals (Callorhinus ursinus) in the Eastern Bering Sea Abstract approved: Redacted for privacy William G. Pearcy This study was conducted to determine the composition and size of prey consumed by northern fur seals (Callorhinus ursinus) in the eastern Bering Sea. Eighty three northern fur seals were collected in the summer and fall of 1981, 1982, and 1985 forexamination of gastrointestinal contents. A total of 139 midwater and bottom trawls were collected to determine the availability of potential prey. Analysis of trawls confirmed that seals are size-selective midwater feeders during their breeding and haul-out season in the eastern Bering Sea. Juvenile walleye pollock and gonatid squid, 5-20cm in body length, were the primary prey, but seal prey varied among years and between nearshore and pelagic sample locations. Interannual variation in body sizes of walleye pollock consumed by seals was related to pollock year class strength. The identification of pollock and gonatid squid as primary fur seal prey in the eastern Bering Sea was consistent with previous reports. However, Pacific herring and capelin, previously considered important fur seal prey werE absent in this study. Feeding Habits of Northern Fur Seals (Callorhinus ursinus) in the Eastern Bering Sea by Elizabeth Hacker Sinclair A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Science Completed December 16, 1988 Commencement June 1989 APPROVED: Redacted for privacy Professor of Oceanography in char.4f major Redacted for privacy Dean of C./lege of Ocean. -

Humboldt Bay Fishes
Humboldt Bay Fishes ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> ·´¯`·._.·´¯`·.. ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> Acknowledgements The Humboldt Bay Harbor District would like to offer our sincere thanks and appreciation to the authors and photographers who have allowed us to use their work in this report. Photography and Illustrations We would like to thank the photographers and illustrators who have so graciously donated the use of their images for this publication. Andrey Dolgor Dan Gotshall Polar Research Institute of Marine Sea Challengers, Inc. Fisheries And Oceanography [email protected] [email protected] Michael Lanboeuf Milton Love [email protected] Marine Science Institute [email protected] Stephen Metherell Jacques Moreau [email protected] [email protected] Bernd Ueberschaer Clinton Bauder [email protected] [email protected] Fish descriptions contained in this report are from: Froese, R. and Pauly, D. Editors. 2003 FishBase. Worldwide Web electronic publication. http://www.fishbase.org/ 13 August 2003 Photographer Fish Photographer Bauder, Clinton wolf-eel Gotshall, Daniel W scalyhead sculpin Bauder, Clinton blackeye goby Gotshall, Daniel W speckled sanddab Bauder, Clinton spotted cusk-eel Gotshall, Daniel W. bocaccio Bauder, Clinton tube-snout Gotshall, Daniel W. brown rockfish Gotshall, Daniel W. yellowtail rockfish Flescher, Don american shad Gotshall, Daniel W. dover sole Flescher, Don stripped bass Gotshall, Daniel W. pacific sanddab Gotshall, Daniel W. kelp greenling Garcia-Franco, Mauricio louvar -
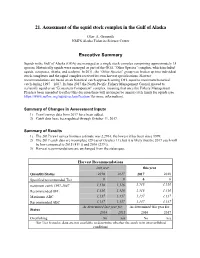
Assessment of the Squid Stock Complex in the Gulf of Alaska
21. Assessment of the squid stock complex in the Gulf of Alaska Olav A. Ormseth NMFS Alaska Fisheries Science Center Executive Summary Squids in the Gulf of Alaska (GOA) are managed as a single stock complex comprising approximately 15 species. Historically squids were managed as part of the GOA “Other Species” complex, which included squids, octopuses, sharks, and sculpins. In 2011, the “Other Species” group was broken up into individual stock complexes and the squid complex received its own harvest specifications. Harvest recommendations are based on an historical catch approach setting OFL equal to maximum historical catch during 1997 – 2007. In June 2017 the North Pacific Fishery Management Council moved to reclassify squid as an “Ecosystem Component” complex, meaning that once the Fishery Management Plan has been amended to reflect this decision there will no longer be annual catch limits for squids (see https://www.npfmc.org/squid-reclassification/ for more information). Summary of Changes in Assessment Inputs 1) Trawl survey data from 2017 have been added. 2) Catch data have been updated through October 11, 2017. Summary of Results 1) The 2017 trawl survey biomass estimate was 2,296 t, the lowest it has been since 1999. 2) The 2017 catch data are incomplete (29 t as of October 11), but it is likely that the 2017 catch will be low compared to 2015 (411 t) and 2016 (239 t). 3) Harvest recommendations are unchanged from the status quo. Harvest Recommendations last year this year Quantity/Status 2016 2017 2017 2018 Specified/recommended -

A Checklist of the Fishes of the Monterey Bay Area Including Elkhorn Slough, the San Lorenzo, Pajaro and Salinas Rivers
f3/oC-4'( Contributions from the Moss Landing Marine Laboratories No. 26 Technical Publication 72-2 CASUC-MLML-TP-72-02 A CHECKLIST OF THE FISHES OF THE MONTEREY BAY AREA INCLUDING ELKHORN SLOUGH, THE SAN LORENZO, PAJARO AND SALINAS RIVERS by Gary E. Kukowski Sea Grant Research Assistant June 1972 LIBRARY Moss L8ndillg ,\:Jrine Laboratories r. O. Box 223 Moss Landing, Calif. 95039 This study was supported by National Sea Grant Program National Oceanic and Atmospheric Administration United States Department of Commerce - Grant No. 2-35137 to Moss Landing Marine Laboratories of the California State University at Fresno, Hayward, Sacramento, San Francisco, and San Jose Dr. Robert E. Arnal, Coordinator , ·./ "':., - 'I." ~:. 1"-"'00 ~~ ~~ IAbm>~toriesi Technical Publication 72-2: A GI-lliGKL.TST OF THE FISHES OF TtlE MONTEREY my Jl.REA INCLUDING mmORH SLOUGH, THE SAN LCRENZO, PAY-ARO AND SALINAS RIVERS .. 1&let~: Page 14 - A1estria§.·~iligtro1ophua - Stone cockscomb - r-m Page 17 - J:,iparis'W10pus." Ribbon' snailt'ish - HE , ,~ ~Ei 31 - AlectrlQ~iu.e,ctro1OphUfi- 87-B9 . .', . ': ". .' Page 31 - Ceb1diehtlrrs rlolaCewi - 89 , Page 35 - Liparis t!01:f-.e - 89 .Qhange: Page 11 - FmWulns parvipin¢.rl, add: Probable misidentification Page 20 - .BathopWuBt.lemin&, change to: .Mhgghilu§. llemipg+ Page 54 - Ji\mdJ11ui~~ add: Probable. misidentifioation Page 60 - Item. number 67, authOr should be .Hubbs, Clark TABLE OF CONTENTS INTRODUCTION 1 AREA OF COVERAGE 1 METHODS OF LITERATURE SEARCH 2 EXPLANATION OF CHECKLIST 2 ACKNOWLEDGEMENTS 4 TABLE 1 -

THE PINNIPEDS of the CALIFORNIA CURRENT California
ANTONELIS AND FISCUS: PINNIPEDS OF THE CALIFORNIA CURRENT CalCOFI Rep., Vol. XXI, 1980 THE PINNIPEDS OF THE CALIFORNIA CURRENT GEORGE A. ANTONELIS. JR. AND CLIFFORD H. FISCUS Marine Mammal Division Northwest and Alaska Fisheries Center National Marine Fisheries Service National Oceanic and Atmospheric Administration 7600 Sand Point Way, N.E. Seattle, WA 981 15 ABSTRACT 10s pequenos peces en 10s cardumenes y peces ana- There are six species of pinnipeds-California sea dromos. Los dos focidos, otra vez con ciertas excep- lion, Zalophus californianus; northern sea lion, Eume- ciones, predan especies diferentes. Aparentemente, el topias jubatus; northern fur seal, Callorhinus ursinus; elefante marino se alimenta en aguas mas profundas que Guadalupe fur seal, Arctocephalus townsendi; harbor la foca peluda, alimentindose de especies demersales seal, Phoca uitulina richardsi; and northern elephant y benticas, y la foca peluda se alimenta de especiesdemer- seal, Mirounga angustirostris-that inhabit the study sales costeras y neriticas, entrando ocasionalmente en rios area of the California Cooperative Oceanic Fisheries y aguas estuarinas haciendopresa de 10s peces anadromos Investigations (CalCOFI). y otros pequeiios peces que entran regularmente en estas The numbers of animals in each population are given; aguas. the size, distribution, and seasonal movements are de- scribed. The known prey species of the pinnipeds are INTRODUCTION listed for each species. The otariids, with certain excep- The California Current, its components, and the Cali- tions, consume the same kinds of prey, although in slight- fornia Cooperative Oceanic Fisheries Investigations ly different amounts. In general they feed most commonly (CalCOFI) station plan have been described many times on the smaller schooling fishes and squids of the epi- in the past and are well known (Kramer et al. -

Guide to the Coastal Marine Fishes of California
STATE OF CALIFORNIA THE RESOURCES AGENCY DEPARTMENT OF FISH AND GAME FISH BULLETIN 157 GUIDE TO THE COASTAL MARINE FISHES OF CALIFORNIA by DANIEL J. MILLER and ROBERT N. LEA Marine Resources Region 1972 ABSTRACT This is a comprehensive identification guide encompassing all shallow marine fishes within California waters. Geographic range limits, maximum size, depth range, a brief color description, and some meristic counts including, if available: fin ray counts, lateral line pores, lateral line scales, gill rakers, and vertebrae are given. Body proportions and shapes are used in the keys and a state- ment concerning the rarity or commonness in California is given for each species. In all, 554 species are described. Three of these have not been re- corded or confirmed as occurring in California waters but are included since they are apt to appear. The remainder have been recorded as occurring in an area between the Mexican and Oregon borders and offshore to at least 50 miles. Five of California species as yet have not been named or described, and ichthyologists studying these new forms have given information on identification to enable inclusion here. A dichotomous key to 144 families includes an outline figure of a repre- sentative for all but two families. Keys are presented for all larger families, and diagnostic features are pointed out on most of the figures. Illustrations are presented for all but eight species. Of the 554 species, 439 are found primarily in depths less than 400 ft., 48 are meso- or bathypelagic species, and 67 are deepwater bottom dwelling forms rarely taken in less than 400 ft.