GPR120 Prevents Colorectal Adenocarcinoma Progression by Sustaining the Mucosal Barrier Integrity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solveig Tosi, Cv Personal Data Born
Solveig Tosi, c.v. Personal data Born: September 20, 1963 in Halden (Norway) Citizenship: Italy Work Address: Department of Earth Science and Environments, University of Pavia, via S. Epifanio 14, 27100 Pavia, Italy Phone: ++39-0382-984870, Fax: ++39-0382-34240 E-mail: [email protected] Fields of Study: fungal ecology and taxonomy, adaptation to cold extreme environments, biological control fungal agents, fungal bioremediation and biofertilization, antifungal activity of natural extracts, science and education. Education and Professional Experience November 2014 – current. Associate Professor, Department of Earth and Environmental Sciences 1998-October 2014. Permanent Researcher, Department of Earth Science and Environments, University of Pavia (Italy) 1991-1998. Technician in the Botanic Garden of Tuscia University (Viterbo, Italy). April 1990 qualification to practise the profession of Biologist. October 1989-October1990. Consultant for a fish farm September1988-February1989. Associate researcher, Biology Institute, Department of Zoology and Marine Chemistry, University of Oslo (Norway), funded by the Research Counsel of Norway. Focus on "Heavy metal concentration in Tmetonix cicada (Crustacea, Amphipoda) and Pandalus borealis (Crustacea, Dacapoda), from Oslo fjord (Norway)", supervisor Prof. M.I.Abdullah. October, 27 1987. B.S. and Masters in Biological Sciences at the University of Rome "La Sapienza" (110/110). Thesis: Teaching: Botany – Course A and B 6 CFU (Biological Science): A.A. 2013/2014 Botany - Course B (Mycology) 3CFU ( Biological Science) .- A.A. 2004/2005; 2005/2006; 2006/2007; 2008/2009; 2009/2010; 2011/2012 Botanic Laboratory 9 CFU (C.L. Experimental and Applied Biology) A. A. 2009/10 ; 2011/2012 Mycology Laboratory 4 CFU (L.S Natural Science) - A.A. -

Breaking up the Global Value Chain: Opportunities and Consequences Advances in International Management
BREAKING UP THE GLOBAL VALUE CHAIN: OPPORTUNITIES AND CONSEQUENCES ADVANCES IN INTERNATIONAL MANAGEMENT Series Editors: (To Volumes 22) Joseph L. C. Cheng and Michael A. Hitt (Volumes 23À30) Timothy M. Devinney, Torben Pedersen and Laszlo Tihanyi Recent Volumes: Volume 14: Edited by M. A. Hitt and J. L. C. Cheng Volume 15: Edited by J. L. C. Cheng and M. A. Hitt Volume 16: Edited by M. A. Hitt and J. L. C. Cheng Volume 17: Edited by Thomas Roehl and Allan Bird Volume 18: Edited by D. L. Shapiro, M. A. Von Glinow and J. L. C. Cheng Volume 19: Edited by M. Javidan, R. M. Steers and M. A. Hitt Volume 20: Edited by Jose´ Antonio Rosa and Madhu Viswanatha Volume 21: Edited by John J. Lawler and Gregory S. Hundley Volume 22: Edited by Joseph L. C. Cheng, Elizabeth Maitland and Stephen Nicholas Volume 23: Edited by Timothy M. Devinney, Torben Pedersen and Laszlo Tihanyi Volume 24: Edited by Christian Geisler Asmussen, Torben Pedersen, Timothy M. Devinney and Laszlo Tihanyi Volume 25: Edited by Laszlo Tihanyi, Timothy M. Devinney and Torben Pedersen Volume 26: Edited by Timothy M. Devinney, Torben Pedersen and Laszlo Tihanyi Volume 27: Edited by Torben Pedersen, Markus Venzin, Timothy M. Devinney and Laszlo Tihanyi Volume 28: Edited by Laszlo Tihanyi, Elitsa R. Banalieva, Timothy M. Devinney and Torben Pedersen Volume 29: Edited by Timothy M. Devinney, Gideon Markman, Laszlo Tihanyi, and Torben Pedersen ADVANCES IN INTERNATIONAL MANAGEMENT VOLUME 30 BREAKING UP THE GLOBAL VALUE CHAIN: OPPORTUNITIES AND CONSEQUENCES EDITED BY TORBEN PEDERSEN Bocconi University, Italy TIMOTHY M. -

CV Francesco Patti Name Francesco Patti Born in Acireale (Italy) 12/06/1958 Scholastic Career II Level Education 1976 Degree Me
Name Francesco Patti Born in Acireale (Italy) 12/06/1958 Scholastic career II level education 1976 Degree Medicine and Surgery 1982 maximum cum laude Post Degree Neurology, University of Catania, 1986 Post Degree Phyisiotherapy, University of Parma, 1990 Work activity 1987-1988 Regional fellowship as Junior Neurologist to study “ Descriptive Neuroepidemiology of most frequent neurological diseases in Sicily”, progetto Regionale 55/P, sponsored by WHO. 1987–1989 Assisting Professor of Neuroendocrinology and Neuroimmunology Scuola di Specializzazione in Neurologia, University of Catania 1991–2000 “CollaboratoreTecnico” Chair of Neurorheabilitation, Institute of Neurological Sciences, University of Catania. 2000–2002 “Tecnico Laureato” (Funzionario Tecnico) Department of Neurological Sciences November 2002 – October 2014 “Ricercatore Confermato” (Aggregate Professor) Clinical Researcher Department of Neurological Sciences, University of Catania. November 2014 - up till now Associate Professor of Neurology, Department of Medical and Surgical Sciences and Advanced Tecnologies G.F. Ingrassia, University of Catania. Clinician profile and activities He is responsible of the tertiary centre of multiple sclerosis at the University of Catania (Italy). The centre follows more than 2500 patients suffering from Multiple Sclerosis and few patients suffering from Devic Disease and Devic Spectrum disorder diseases. The centre follows also patients suffering from ALS (currently 100 patients) and other people with different forms of spasticity, offering them with a multidisciplinary approach every kind of Pag. 1 of 3 CV Francesco Patti You created this PDF from an application that is not licensed to print to novaPDF printer (http://www.novapdf.com) assistance. Scientific activity Research Interests ; Preclinical (1981-1988) ; Neurochemistry, Neuroendocrinolgy, Neuropsycopharmacology ; Clinical: (1989-current) ; Neuroepidemiology ; Clinical Immunology ; Quality of Life ; Neurorehabilitation ; Multiple Sclerosis. -
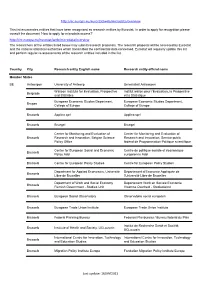
Eurostat: Recognized Research Entity
http://ec.europa.eu/eurostat/web/microdata/overview This list enumerates entities that have been recognised as research entities by Eurostat. In order to apply for recognition please consult the document 'How to apply for microdata access?' http://ec.europa.eu/eurostat/web/microdata/overview The researchers of the entities listed below may submit research proposals. The research proposal will be assessed by Eurostat and the national statistical authorities which transmitted the confidential data concerned. Eurostat will regularly update this list and perform regular re-assessments of the research entities included in the list. Country City Research entity English name Research entity official name Member States BE Antwerpen University of Antwerp Universiteit Antwerpen Walloon Institute for Evaluation, Prospective Institut wallon pour l'Evaluation, la Prospective Belgrade and Statistics et la Statistique European Economic Studies Department, European Economic Studies Department, Bruges College of Europe College of Europe Brussels Applica sprl Applica sprl Brussels Bruegel Bruegel Center for Monitoring and Evaluation of Center for Monitoring and Evaluation of Brussels Research and Innovation, Belgian Science Research and Innovation, Service public Policy Office fédéral de Programmation Politique scientifique Centre for European Social and Economic Centre de politique sociale et économique Brussels Policy Asbl européenne Asbl Brussels Centre for European Policy Studies Centre for European Policy Studies Department for Applied Economics, -

WUDR Biology
www.cicerobook.com Biology 2021 TOP-500 Double RankPro 2021 represents universities in groups according to the average value of their ranks in the TOP 500 of university rankings published in a 2020 World University Country Number of universities Rank by countries 1-10 California Institute of Technology Caltech USA 1-10 Harvard University USA Australia 16 1-10 Imperial College London United Kingdom Austria 2 1-10 Massachusetts Institute of Technology USA Belgium 7 1-10 Stanford University USA Brazil 1 1-10 University College London United Kingdom Canada 12 1-10 University of California, Berkeley USA China 14 1-10 University of Cambridge United Kingdom Czech Republic 1 1-10 University of Oxford United Kingdom Denmark 4 1-10 Yale University USA Estonia 1 11-20 Columbia University USA Finland 4 11-20 Cornell University USA France 9 11-20 ETH Zürich-Swiss Federal Institute of Technology Zurich Switzerland Germany 26 11-20 Johns Hopkins University USA Greece 1 11-20 Princeton University USA Hong Kong 3 11-20 University of California, Los Angeles USA Ireland 4 11-20 University of California, San Diego USA Israel 4 11-20 University of Pennsylvania USA Italy 11 11-20 University of Toronto Canada Japan 6 11-20 University of Washington USA Netherlands 9 21-30 Duke University USA New Zealand 2 21-30 Karolinska Institutet Sweden Norway 3 21-30 Kyoto University Japan Portugal 2 21-30 Ludwig-Maximilians University of Munich Germany Rep.Korea 5 21-30 National University of Singapore Singapore Saudi Arabia 2 21-30 New York University USA Singapore 2 21-30 -

Pier Giovanni Bissiri – Curriculum Vitae
Department of Statistical Sciences "Paolo Fortunati" Via Belle Arti 41 40126 Bologna Pier Giovanni Bissiri T +39 0512098279 B [email protected] Curriculum Vitae ORCID: https://orcid.org/0000-0003-3769-6649 Present position 11/2019– Tenure–track assistant professor (senior), at Department of Statistical Sciences, University of Bologna, Bologna, Italy. Previous positions 10/2017–11/2019 Research associate, at School of Mathematics, Statistics and Physics, New- castle University, Newcastle, UK, supervisor: prof. Emilio Porcu . topic: Positive definite functions in geostatistics 01/2015–09/2017 Postdoctoral position (renewed on 01/01/2017), at the Dep. of Econo- mics, Management and Statistics, University of Milano–Bicocca, Milan, Italy, supervisor: prof. A. Ongaro. topic: discrete Bayesian nonparametric models 04/2014–12/2014 Postdoctoral position, at IMATI, Institute of Applied Mathematics and Information Technology of the National Research Council (CNR), Milan, Italy. 01/2014–03/2014 Collaborator, at the Department of Economics, Management and Statistics of the University of Milano–Bicocca, Milan, Italy. 01/2010–12/2013 Postdoctoral position (renewed on 01/01/2012), at the Department of Economics, Management and Statistics of the University of Milano–Bicocca, Milan, Italy. topic: Non parametric Bayesian inference 12/2007–11/2009 Two years research grant ‘Master and Back’, funded by the Region of Sardinia, Italy, at the Department of Mathematics of the University of Cagliari, Italy. Qualification for professorship 10/02/2014 National scientific qualification to function as associate professor in Italian Universities in Statistics, obtained for the period 10/02/2014–10/02/2020, and renewed until 26/11/2020. https://asn.cineca.it/ministero.php/ public/esito/settore/13%252FD1/fascia/2 Education 19/1/2007 PhD in “Mathematics and Statistics”, Department of Mathematics at the University of Pavia, Pavia, Italy, Curriculum: Probability and Statistics. -

International Students Handbook
1 www.unipv.eu Dear Student, Welcome to the University of Pavia! We are glad you have chosen to study with us and we hope this handbook will help you learn more about life at our campus. Please read it carefully before you ar- rive and keep it ready at hand for future reference during your stay. Our aim is to provide you with practical information about life as a new interna- tional/exchange student and as a new member of Pavia’s academic commu- nity. Please note this booklet is for infor- mation purposes only. We make ev- ery effort to ensure that it is accurate at press time. However, the university shall not be responsible for any errors or omissions. 2 3 International Students Handbook www.unipv.eu URBAN BUS Pavia local bus company is called How to reach Pavia Autoguidovie. You can buy tickets on and move around the city board, at news stand or directly on the app “Autoguidovie”. The app is free and available in several languages. On the app Pavia is located 30 km south of Milan, ORIO AL SERIO AIRPORT (BGY) you can plan your journey and check the to which is connected by trains every Several buses connect this airport to Milan bus schedule for each bus stop. UNIPV 20 minutes. The University has two central railway station (around 5 €). From and Autoguidovie offer all students a main campuses: Humanities and Social Milan Central railway station it is then bus pass for the whole academic year at sciences in the city center, the scientific possible to take a train to Pavia (4 €). -

Folder Ciiscam Solo Inglese
Department of Ecology and Economic Sustainable Development Under the High Patronage of The President of the Republic of Italy ITALIAN OFFICIAL WORLD FOOD DAY CELEBRATIONS 2007 The Right to Food Under the Patronage of The City of Viterbo The Province of Viterbo The Agriculture Commission of the Region of Lazio The Chair of the Council of the Lazio Region The Ministry of Agriculture 1° INTERNATIONAL C.I.I.S.C.A.M.CONFERENCE IINTERNATIONAL INTER-UNIVERSITY CENTRE FOR MEDITERRANEAN FOOD CULTURE STUDIES New Frontiers in the Mediterranean for Food Security Mediterranean Diet and Well Being Food Safety and Quality Biodiversity and Nutrition 4-5 December 2007 Rector Hall, via Santa Maria in Gradi, 4 Viterbo in cooperation with FORUM ON National Institute for Research Nutrition and Consumer MEDITERRANEAN Italian Official Celebrations on Food and Nutrition Protection Division FOOD CULTURES WORLD FOOD DAY 2007 1° INTERNATIONAL CIISCAM CONFERENCE CIISCAM INTERUNIVERSITY INTERNATIONAL CENTRE FOR MEDITERRANEAN FOOD CULTURES STUDIES The CIISCAM - Interuniversity International Centre for OBJECTIVES: Mediterranean Food Cultures Studies - has been esta- - To promote, realize and coordinate researches in the blished on 25 July 2006 by the Sapienza University of field of food science, with particular regards to Rome, the University of Calabria, the University of Gran Mediterranean food cultures; Canaria, the University of Parma and the University of - To foster cooperation among participant universities Tuscia. Its administrative office is at the Sapienza -

Welcome Package for Erasmus and International Students
WELCOME PACKAGE FOR ERASMUS AND INTERNATIONAL STUDENTS University of Parma - Main Building 2 WELCOME TO THE UNIVERSITY OF PARMA We are delighted that you have chosen to study at the University of Parma. We hope that your time here will be challenging, academically rewar- ding and enjoyable. This Welcome Guide is designed to help you manage the process of planning and moving to Parma for your studying experience. It provi- des you with instructions and guidance on visa application, registra- tion procedures, admission requirements for the courses and on the services and facilities at your disposal. Moreover, this booklet includes practical information for your arrival and your stay in Parma, as well as maps and important contact details. We have also added a specific section on Parma, containing handy tips on what to see and do in your free time. We look forward to meeting and welcoming you to the University of Parma. The University of Parma has been awarded the ECTS Label twice in a row, for the period 2009-2013 and 2013-2016. 3 Table of CONTENTS Why choosing the University of Parma 6 APPLYING FOR UNIPR 9 How to apply - Exchange Students 10 Before your arrival - Exchange Students 12 On arrival - Exchange Students 12 At the end of your exchange period 14 How to apply - International Degree-seeking Students 16 Application procedure 20 Visa application 28 Registration to the town council records 32 Residence permit 33 Italian tax code - codice fiscale 35 STUDYING AT UNIPR 36 Academic calendar 37 The University Departments 38 Course catalogue -

Unveiling Role of Sphingosine-1-Phosphate Receptor 2 As a Brake of Epithelial Stem Cell Proliferation and a Tumor Suppressor in Colorectal Cancer
Unveiling role of Sphingosine-1-phosphate receptor 2 as a brake of epithelial stem cell proliferation and a tumor suppressor in colorectal cancer Luciana Petti Humanitas Clinical and Research Center-IRCCS Giulia Rizzo Humanitas University Federica Rubbino Humanitas University Sudharshan Elangovan Humanitas University Piergiuseppe Colombo Humanitas Clinical and Research Center-IRRCS Restelli Silvia Humanitas University Andrea Piontini Humanitas University Vincenzo Arena Policlinico Universitario Agostino Gemelli Michele Carvello Humanitas Clinical and Research Center-IRCCS Barbara Romano Universita degli Studi di Napoli Federico II Dipartimento di Medicina Clinica e Chirurgia Tommaso Cavalleri Humanitas Clinical and Research Center-IRCCS Achille Anselmo Humanitas Clinical and Research Center-IRCCS Federica Ungaro Humanitas University Silvia D’Alessio Humanitas University Antonino Spinelli Humanitas University Sanja Stifter Page 1/28 University of Rijeka Fabio Grizzi Humanitas Clinical and Research Center-IRCCS Alessandro Sgambato Istituto di Ricovero e Cura a Carattere Scientico Centro di Riferimento Oncologico della Basilicata Silvio Danese Humanitas University Luigi Laghi Universita degli Studi di Parma Alberto Malesci Humanitas University STEFANIA VETRANO ( [email protected] ) Humanitas University Research Keywords: colorectal cancer, Lgr5, S1PR2, PTEN, epithelial proliferation Posted Date: October 13th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-56319/v2 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published on November 23rd, 2020. See the published version at https://doi.org/10.1186/s13046-020-01740-6. Page 2/28 Abstract Background. Sphingosine-1-phosphate receptor 2 (S1PR2) mediates pleiotropic functions encompassing cell proliferation, survival, and migration, which become collectively de-regulated in cancer. -

Alessandro Tasora
Curriculum Vitae Alessandro Tasora Born 6-3-1971 in Milano. Fiscal code: TSRLSN71C06F205H v. Avesella 26, 40121 Bologna, ITALY [email protected] http://projectchrono.org/tasora/ http://www.chronoengine.info WORK EXPERIENCE 2014- Associate Professor, at the Department of Industrial Engineering, University of Parma, Italy. 2002-2014 Assistant Professor, at the Department of Industrial Engineering, University of Parma, Italy. 1998-2002 Researcher at the Dipartimento di Sistemi di Trasporto e Movimentazione and Dipartimento di Elettrotecnica, at Politecnico di Milano, Italy. EDUCATION Mechanical Engineering at the Politecnico di Milano, M.Sc. July 1998. Thesis: " Simulazione multibody mediante algebra dei quaternioni " (“Multibody simulation by means of quaternion algebra”). Italian State Certification for enabling the public profession in Engineering, 1999. ACADEMIC APPOINTMENTS Associate Professor, Università degli Studi di Parma, area ING-IND-13 09/A2 (Applied Mechanics), since 1/10/2014. Honorary Associate at the University of Wisconsin Madison, USA, since 2009. OTHER ACADEMIC TITLES AND ORGANIZING ACTIVITIES National Scientific Qualification ASN 2016, enabling to apply for a university Full Professor position. Deputy of the Università degli Studi di Parma at the ITS Maker Foundation, since 3/11/2016 Member of the Committee for the PhD in Industrial Engineering, at the Università degli Studi di Parma, since 2002. Member of the Committee for the assessment of PhD students in Industrial Engineering at the Università degli Studi di Parma, since 2013. Member of the Exams Committee and Opponent for the PhD theses at Technischen Universität Kaiserslautern, Germany (24/7/2015), Member of the Exams Committee and Opponent for the PhD theses at the Politecnico di Milano, Aerospace Engineering PhD (17/4/2009), Università degli Studi di Bergamo (14/4/2010, 26/4/2012), Università di Roma La Sapienza (9/11/2012), Politecnico di Milano, Mechanical Engineering (30/3/2015). -

Prof. Matteo Giovanni Della Porta
Curriculum Vitae Replace with First name(s) Surname(s) PERSONAL INFORMATION Prof. Matteo Giovanni Della Porta Cancer Center - IRCCS Humanitas Research Hospital & Humanitas University Via Manzoni, 113 - 20089 Rozzano - Milan, Italy Phone +39 02 8224 7668 Fax + 39 02 8224 4592 Mail [email protected] Web mdellaporta.com Date of birth 29/05/1974 | Nationality Italian Head, Leukemia Unit - Cancer Center - IRCCS Humanitas Research Hospital Associate professor of Hematology - Humanitas University POSITION Via Manzoni, 113 - 20089 Rozzano - Milan, Italy www.humanitas.it www.hunimed.eu WORK EXPERIENCE 2016- Head, Leukemia Unit - Cancer Center IRCCS Humanitas Research Hospital Milan, Italy & Associate Professor of Hematology – Humanitas University, Milan Italy 2014-2016 Associate Professor of Clinical Oncology – University of Pavia, Italy 2008-2015 Assistant Professor of Clinical Oncology & Consultant Hematologist, University of Pavia Italy & Department of Hematology IRCCS Policlinico San Matteo, Pavia Italy EDUCATION AND TRAINING 2003 Board Certification in Hematology - University of Ferrara, Italy 1999 Medical Degree - University of Pavia, Italy PERSONAL SKILLS Current research interests mainly concern myeloid malignancies (myelodysplastic syndromes, MDS acute myeloid leukemias AML, myeloproliferative neoplasms, MPN) These investigations led to the definition of specific gene expression profiles in myelodysplastic syndromes [Blood. 2006;108(1):337-45 and Leukemia. 2010;24(4):756-64 and J Clin Oncol 2013, J Clin Oncol. 2013 Oct 1;31(28):3557-64], to the identification of the molecular basis of refractory anemia with ringed sideroblasts associated with marked thrombocytosis [Blood. 2009;114(17):3538-45], and to the development of the WPSS [J Clin Oncol. 2007;25(23):3503-10, Blood.