(Plerogyra Sinuosa) with 'Photosynthetic Organs'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Resurrecting a Subgenus to Genus: Molecular Phylogeny of Euphyllia and Fimbriaphyllia (Order Scleractinia; Family Euphylliidae; Clade V)
Resurrecting a subgenus to genus: molecular phylogeny of Euphyllia and Fimbriaphyllia (order Scleractinia; family Euphylliidae; clade V) Katrina S. Luzon1,2,3,*, Mei-Fang Lin4,5,6,*, Ma. Carmen A. Ablan Lagman1,7, Wilfredo Roehl Y. Licuanan1,2,3 and Chaolun Allen Chen4,8,9,* 1 Biology Department, De La Salle University, Manila, Philippines 2 Shields Ocean Research (SHORE) Center, De La Salle University, Manila, Philippines 3 The Marine Science Institute, University of the Philippines, Quezon City, Philippines 4 Biodiversity Research Center, Academia Sinica, Taipei, Taiwan 5 Department of Molecular and Cell Biology, James Cook University, Townsville, Australia 6 Evolutionary Neurobiology Unit, Okinawa Institute of Science and Technology Graduate University, Okinawa, Japan 7 Center for Natural Sciences and Environmental Research (CENSER), De La Salle University, Manila, Philippines 8 Taiwan International Graduate Program-Biodiversity, Academia Sinica, Taipei, Taiwan 9 Institute of Oceanography, National Taiwan University, Taipei, Taiwan * These authors contributed equally to this work. ABSTRACT Background. The corallum is crucial in building coral reefs and in diagnosing systematic relationships in the order Scleractinia. However, molecular phylogenetic analyses revealed a paraphyly in a majority of traditional families and genera among Scleractinia showing that other biological attributes of the coral, such as polyp morphology and reproductive traits, are underutilized. Among scleractinian genera, the Euphyllia, with nine nominal species in the Indo-Pacific region, is one of the groups Submitted 30 May 2017 that await phylogenetic resolution. Multiple genetic markers were used to construct Accepted 31 October 2017 Published 4 December 2017 the phylogeny of six Euphyllia species, namely E. ancora, E. divisa, E. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

AC29 Doc13.3 A2
AC29 Doc. 13.3 Annex 2 Selection of species for inclusion in the Review of Significant Trade following CoP17 To comply with Stage 1 a) of Resolution Conf. 12.8 (Rev. CoP17) and the Guidance regarding the selection of species/country combinations outlined in Annex 2 of the Resolution, the UN Environment World Conservation Monitoring Centre (UNEP-WCMC) has produced an extended analysis to assist the Animals Committee with their work in selecting species for inclusion in the Review of Significant Trade following CoP17. A summary output, providing trade in wild, ranched, source unknown and trade without a source specified over the five most recent years (2011-2015), to accompany this analysis is provided in AC29 Doc. 13.3, Annex 1. The methodology for the extended analysis was discussed by the 2nd meeting of the Advisory Working Group (AWG) of the Evaluation of the Review of Significant Trade (Shepherdstown, 2015). The AWG concluded that three criteria should be retained within the methodology (“high volume trade/high volume trade for globally threatened species”, “sharp increase in trade” and “endangered species in trade”), but that two previously used criteria added little value to the prioritisation exercise (“high variability in trade” and “overall increase/overall decrease in trade”). In addition, it was agreed to refine the methodology for “High Volume” trade to ensure that thresholds are set at a fine taxonomic resolution (order level) to ensure representation for all taxonomic orders. It was also agreed to include analysis of “Sharp Increase” in trade at the country level, as well as at the global level. -

Vir Smiti Spec. Nov., a New Scleractinian Associated Pontoniine Shrimp (Crustacea: Decapoda: Palaemonidae) from the Indo-West Pacifi C
Vir smiti spec. nov., a new scleractinian associated pontoniine shrimp (Crustacea: Decapoda: Palaemonidae) from the Indo-West Pacifi c C.H.J.M. Fransen & L.B. Holthuis Fransen, C.H.J.M. & L.B. Holthuis. Vir smiti spec. nov., a new scleractinian associated pontoniine shrimp (Crustacea: Decapoda: Palaemonidae) from the Indo-West Pacifi c. Zool. Med. Leiden 81 (4) 8.vi.2007: 101-114, fi gs. 1-37.— ISSN 0024-0672. Nationaal Natuurhistorisch Museum, Postbus 9517, 2300 RA Leiden, The Netherlands (E-mail: fransen@ naturalis.nnm.nl). Key words: Crustacea; Decapoda; Caridea; Palaemonidae; Vir smiti; new species; scleractinian associate; Indo-West Pacifi c. A new species of scleractinian associated pontoniine shrimp, Vir smiti spec. nov., is described and illus- trated on the basis of specimens collected throughout the Indo-West Pacifi c. Introduction The genus Vir was erected by Holthuis (1952) to accommodate Palaemonella orientalis Dana, 1852, which differs from Palaemonella species in lacking an hepatic spine. Vir orien- talis has been recorded from Pocillopora damicornis (Linnaeus, 1758) and several species of Acropora Oken, 1815. In recent years, several morphologically very similar new species of Vir have been described from various scleractinian corals within the family Euphyllii- dae Veron, 2000: V. philippinensis Bruce & Svoboda, 1984 and V. colemani Bruce, 2003, both mainly recorded from the genus Plerogyra Milne Edwards & Haime, 1848; V. euphyllius Marin & Anker, 2005 from Euphyllia cf. divisa and V. pareuphyllius Marin & Anker, 2005 from Euphyllia cf. parancora Veron, 1990. On the basis of more material, Marin (in press) recognized Vir pareuphyllius as a junior synonym of V. -
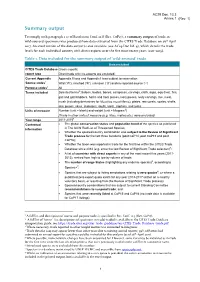
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g. -

Conservation of Reef Corals in the South China Sea Based on Species and Evolutionary Diversity
Biodivers Conserv DOI 10.1007/s10531-016-1052-7 ORIGINAL PAPER Conservation of reef corals in the South China Sea based on species and evolutionary diversity 1 2 3 Danwei Huang • Bert W. Hoeksema • Yang Amri Affendi • 4 5,6 7,8 Put O. Ang • Chaolun A. Chen • Hui Huang • 9 10 David J. W. Lane • Wilfredo Y. Licuanan • 11 12 13 Ouk Vibol • Si Tuan Vo • Thamasak Yeemin • Loke Ming Chou1 Received: 7 August 2015 / Revised: 18 January 2016 / Accepted: 21 January 2016 Ó Springer Science+Business Media Dordrecht 2016 Abstract The South China Sea in the Central Indo-Pacific is a large semi-enclosed marine region that supports an extraordinary diversity of coral reef organisms (including stony corals), which varies spatially across the region. While one-third of the world’s reef corals are known to face heightened extinction risk from global climate and local impacts, prospects for the coral fauna in the South China Sea region amidst these threats remain poorly understood. In this study, we analyse coral species richness, rarity, and phylogenetic Communicated by Dirk Sven Schmeller. Electronic supplementary material The online version of this article (doi:10.1007/s10531-016-1052-7) contains supplementary material, which is available to authorized users. & Danwei Huang [email protected] 1 Department of Biological Sciences and Tropical Marine Science Institute, National University of Singapore, Singapore 117543, Singapore 2 Naturalis Biodiversity Center, PO Box 9517, 2300 RA Leiden, The Netherlands 3 Institute of Biological Sciences, Faculty of -

Factors Controlling the Expansion Behavior of Favia Favus (Cnidaria: Scleractinia): Effects of Light, Flow, and Planktonic Prey
Reference: Biol. Bull. 200: 118–126. (April 2001) Factors Controlling the Expansion Behavior of Favia favus (Cnidaria: Scleractinia): Effects of Light, Flow, and Planktonic Prey O. LEVY*, L. MIZRAHI, N. E. CHADWICK-FURMAN, AND Y. ACHITUV Faculty of Life Sciences, Bar-Ilan University, Ramat Gan 52900, Israel Abstract. Colonies of the massive stony coral Favia favus Corals that expand tentacles at night remain open until were exposed to different flow speeds and levels of light, dawn, but a beam of light or mechanical stimulation may and to the addition of zooplankton prey. The relative im- cause them to contract immediately (Abe, 1939). Sea anem- portance of each factor in controlling polyp expansion be- ones show a similar range of behavior. Structures containing havior was tested. The coral polyps fully expanded when high densities of zooxanthellae (such as pseudotentacles and they were exposed to low light intensity (0–40 mol mϪ2 column vesicles) may expand under conditions favorable sϪ1) and high flow speed (15 cm sϪ1), regardless of prey for photosynthesis, whereas structures that contain few or presence. They also partially expanded under low and me- no zooxanthellae (such as feeding tentacles) contract under dium light (40–80 mol mϪ2 sϪ1) at medium flow speed these conditions (Sebens and DeRiemer, 1977). In the stony (10 cm sϪ1). The corals expanded their polyps only when coral Plerogyra sinuosa, vesicles with high zooxanthellae they were exposed to light levels below compensation irra- density expand only during daytime, and feeding tentacles diance (Icom: light level at which photosynthesis ϭ respira- expand at night (Vareschi and Fricke, 1986). -

(Crustacea: Decapoda) from the Andaman and Nicobar Islands, India J.S
Scholars Academic Journal of Biosciences (SAJB) ISSN 2321-6883 (Online) Sch. Acad. J. Biosci., 2015; 3(1B):113-119 ISSN 2347-9515 (Print) ©Scholars Academic and Scientific Publisher (An International Publisher for Academic and Scientific Resources) www.saspublisher.com Research Article A report on some symbiotic shrimps (Crustacea: Decapoda) from the Andaman and Nicobar Islands, India J.S. Yogesh Kumar*, C. Raghunathan, K. Venkataraman1 Zoological Survey of India, Andaman and Nicobar Regional Centre, National Coral Reef Research Institute, Port Blair- 744102, Andaman & Nicobar Islands, India. 1Zoological Survey of India, M-Block, New Alipore, Kolkata – 700053, India. *Corresponding author J.S. Yogesh Kumar Email: [email protected] Abstract: Symbiotic shrimps (crustacean) were searched for on invertebrates such as sea anemones (Actiniaria), Hard corals (Sclerectinia), horny coral (Gorgonaria), black coral (Antipatharia), Coriocella nigra (Mollusca), Star fish and sea urchins (Echinodermata) in the Andaman and Nicobar Islands. Fourteen species of invertebrate associated shrimps belonging to 10 genera and 6 families were recorded from this region and two invertebrate hosts (Coriocella nigra Blainville, 1824 and Actinodendron glomeratum Haddon, 1898) were also newly observed in the Andaman and Nicobar Islands as well as in India. Keywords: Decapoda, symbiosis, Invertebrate, Andaman and Nicobar Islands. INTRODUCTION ranges of 5 – 40 m. The geographic locations of the A symbiotic life style is one of the greatest sampling sites are represented in table – 1. The shrimps environmental adaptations of marine crustaceans [1]. were observed along with Echinodermata, Mollusca, Although most symbiotic decapods inhabit their host as Actiniaria, Sclerectinia and Antipatharia of the sub tidal solitary individuals or as a mated pair [2], there are also region. -

A Review of Marine Viruses in Coral Ecosystem
Journal of Marine Science and Engineering Review A Review of Marine Viruses in Coral Ecosystem Logajothiswaran Ambalavanan 1 , Shumpei Iehata 1, Rosanne Fletcher 1, Emylia H. Stevens 1 and Sandra C. Zainathan 1,2,* 1 Faculty of Fisheries and Food Sciences, University Malaysia Terengganu, Kuala Nerus 21030, Terengganu, Malaysia; [email protected] (L.A.); [email protected] (S.I.); rosannefl[email protected] (R.F.); [email protected] (E.H.S.) 2 Institute of Marine Biotechnology, University Malaysia Terengganu, Kuala Nerus 21030, Terengganu, Malaysia * Correspondence: [email protected]; Tel.: +60-179261392 Abstract: Coral reefs are among the most biodiverse biological systems on earth. Corals are classified as marine invertebrates and filter the surrounding food and other particles in seawater, including pathogens such as viruses. Viruses act as both pathogen and symbiont for metazoans. Marine viruses that are abundant in the ocean are mostly single-, double stranded DNA and single-, double stranded RNA viruses. These discoveries were made via advanced identification methods which have detected their presence in coral reef ecosystems including PCR analyses, metagenomic analyses, transcriptomic analyses and electron microscopy. This review discusses the discovery of viruses in the marine environment and their hosts, viral diversity in corals, presence of virus in corallivorous fish communities in reef ecosystems, detection methods, and occurrence of marine viral communities in marine sponges. Keywords: coral ecosystem; viral communities; corals; corallivorous fish; marine sponges; detec- tion method Citation: Ambalavanan, L.; Iehata, S.; Fletcher, R.; Stevens, E.H.; Zainathan, S.C. A Review of Marine Viruses in Coral Ecosystem. J. Mar. Sci. Eng. 1. -

Tonga SUMA Report
BIOPHYSICALLY SPECIAL, UNIQUE MARINE AREAS OF TONGA EFFECTIVE MANAGEMENT Marine and coastal ecosystems of the Pacific Ocean provide benefits for all people in and beyond the region. To better understand and improve the effective management of these values on the ground, Pacific Island Countries are increasingly building institutional and personal capacities for Blue Planning. But there is no need to reinvent the wheel, when learning from experiences of centuries of traditional management in Pacific Island Countries. Coupled with scientific approaches these experiences can strengthen effective management of the region’s rich natural capital, if lessons learnt are shared. The MACBIO project collaborates with national and regional stakeholders towards documenting effective approaches to sustainable marine resource management and conservation. The project encourages and supports stakeholders to share tried and tested concepts and instruments more widely throughout partner countries and the Oceania region. This report outlines the process undertaken to define and describe the special, unique marine areas of Tonga. These special, unique marine areas provide an important input to decisions about, for example, permits, licences, EIAs and where to place different types of marine protected areas, locally managed marine areas and Community Conservation Areas in Tonga. For a copy of all reports and communication material please visit www.macbio-pacific.info. MARINE ECOSYSTEM MARINE SPATIAL PLANNING EFFECTIVE MANAGEMENT SERVICE VALUATION BIOPHYSICALLY SPECIAL, UNIQUE MARINE AREAS OF TONGA AUTHORS: Ceccarelli DM1, Wendt H2, Matoto AL3, Fonua E3, Fernandes L2 SUGGESTED CITATION: Ceccarelli DM, Wendt H, Matoto AL, Fonua E and Fernandes L (2017) Biophysically special, unique marine areas of Tonga. MACBIO (GIZ, IUCN, SPREP), Suva. -

Crustacea: Decapoda: Palaemonidae)
Arthropoda Selecta 14 (2): 117128 © ARTHROPODA SELECTA, 2005 Two new species of the genus Vir Holthuis, 1952 from Vietnam (Crustacea: Decapoda: Palaemonidae) Äâà íîâûõ âèäà ðîäà Vir Holthuis, 1952 èç Âüåòíàìà (Crustacea: Decapoda: Palaemonidae) I.N. Marin1 & A. Anker2 È.Í. Ìàðèí, À. Àíêåð 1 Laboratory of Ecology and Morphology of Marine Invertebrates, A. N. Severtzov Institute of Ecology and Evolution, Russian Academy of Science, Leninsky prospect, 33, 119071 Moscow Russia. E-mail: [email protected] Ëàáîðàòîðèÿ ýêîëîãèè è ìîðôîëîãèè ìîðñêèõ áåñïîçâîíî÷íûõ, Èíñòèòóò ïðîáëåì ýêîëîãèè è ýâîëþöèè èì. À.Í. Ñåâåðöîâà ÐÀÍ, Ëåíèíñêèé ïðîñïåêò, 33, Ìîñêâà 117071 Ðîññèÿ. 2 Department of Biological Sciences, University of Alberta, Edmonton T6G 2E9 Canada. E-mail: [email protected] KEY WORDS: Palemonidae, Vir, new species, bubble coral, Euphyllia, Caryophyllidae, host specificity, Vietnam. ÊËÞ×ÅÂÛÅ ÑËÎÂÀ: Palemonidae, Vir, íîâûé âèä, Euphyllia, Caryophyllidae, ñïåöèôè÷íîñòü ê õîçÿèíó, Âüåòíàì. ABSTRACT. Vir euphyllius, sp.n., and Vir pareu- photographers. These fragile-looking, mostly transpar- phylius, sp.n., are described on the basis of several ent shrimps are among the most frequently photographed specimens of both sexes collected from caryophillid Indo-West Pacific marine shrimps. Previously, only corals Euphyllia spp. in Nhatrang Bay, Vietnam. The three species were known in this genus: the type spe- new species differ from V. philippinensis Bruce & cies, V. orientalis (Dana, 1852), V. philippinensis Bruce Svoboda and V. colemani Bruce by the less conspicu- & Svoboda, 1984, and the recently described V. cole- ous colour pattern and a combination of morphological mani Bruce, 2003. During an extensive sampling of characters, and from the type species, V.