A New Player Influencing Seed Longevity and Dormancy In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anti-Inflammatory Role of Curcumin in LPS Treated A549 Cells at Global Proteome Level and on Mycobacterial Infection
Anti-inflammatory Role of Curcumin in LPS Treated A549 cells at Global Proteome level and on Mycobacterial infection. Suchita Singh1,+, Rakesh Arya2,3,+, Rhishikesh R Bargaje1, Mrinal Kumar Das2,4, Subia Akram2, Hossain Md. Faruquee2,5, Rajendra Kumar Behera3, Ranjan Kumar Nanda2,*, Anurag Agrawal1 1Center of Excellence for Translational Research in Asthma and Lung Disease, CSIR- Institute of Genomics and Integrative Biology, New Delhi, 110025, India. 2Translational Health Group, International Centre for Genetic Engineering and Biotechnology, New Delhi, 110067, India. 3School of Life Sciences, Sambalpur University, Jyoti Vihar, Sambalpur, Orissa, 768019, India. 4Department of Respiratory Sciences, #211, Maurice Shock Building, University of Leicester, LE1 9HN 5Department of Biotechnology and Genetic Engineering, Islamic University, Kushtia- 7003, Bangladesh. +Contributed equally for this work. S-1 70 G1 S 60 G2/M 50 40 30 % of cells 20 10 0 CURI LPSI LPSCUR Figure S1: Effect of curcumin and/or LPS treatment on A549 cell viability A549 cells were treated with curcumin (10 µM) and/or LPS or 1 µg/ml for the indicated times and after fixation were stained with propidium iodide and Annexin V-FITC. The DNA contents were determined by flow cytometry to calculate percentage of cells present in each phase of the cell cycle (G1, S and G2/M) using Flowing analysis software. S-2 Figure S2: Total proteins identified in all the three experiments and their distribution betwee curcumin and/or LPS treated conditions. The proteins showing differential expressions (log2 fold change≥2) in these experiments were presented in the venn diagram and certain number of proteins are common in all three experiments. -

Noelia Díaz Blanco
Effects of environmental factors on the gonadal transcriptome of European sea bass (Dicentrarchus labrax), juvenile growth and sex ratios Noelia Díaz Blanco Ph.D. thesis 2014 Submitted in partial fulfillment of the requirements for the Ph.D. degree from the Universitat Pompeu Fabra (UPF). This work has been carried out at the Group of Biology of Reproduction (GBR), at the Department of Renewable Marine Resources of the Institute of Marine Sciences (ICM-CSIC). Thesis supervisor: Dr. Francesc Piferrer Professor d’Investigació Institut de Ciències del Mar (ICM-CSIC) i ii A mis padres A Xavi iii iv Acknowledgements This thesis has been made possible by the support of many people who in one way or another, many times unknowingly, gave me the strength to overcome this "long and winding road". First of all, I would like to thank my supervisor, Dr. Francesc Piferrer, for his patience, guidance and wise advice throughout all this Ph.D. experience. But above all, for the trust he placed on me almost seven years ago when he offered me the opportunity to be part of his team. Thanks also for teaching me how to question always everything, for sharing with me your enthusiasm for science and for giving me the opportunity of learning from you by participating in many projects, collaborations and scientific meetings. I am also thankful to my colleagues (former and present Group of Biology of Reproduction members) for your support and encouragement throughout this journey. To the “exGBRs”, thanks for helping me with my first steps into this world. Working as an undergrad with you Dr. -

Prioritization of Metabolic Genes As Novel Therapeutic Targets in Estrogen-Receptor Negative Breast Tumors Using Multi-Omics Data and Text Mining
www.oncotarget.com Oncotarget, 2019, Vol. 10, (No. 39), pp: 3894-3909 Research Paper Prioritization of metabolic genes as novel therapeutic targets in estrogen-receptor negative breast tumors using multi-omics data and text mining Dinesh Kumar Barupal1,*, Bei Gao1,*, Jan Budczies2, Brett S. Phinney4, Bertrand Perroud4, Carsten Denkert2,3 and Oliver Fiehn1 1West Coast Metabolomics Center, University of California, Davis, CA, USA 2Institute of Pathology, Charité University Hospital, Berlin, Germany 3German Institute of Pathology, Philipps-University Marburg, Marburg, Germany 4UC Davis Genome Center, University of California, Davis, CA, USA *Co-first authors and contributed equally to this work Correspondence to: Oliver Fiehn, email: [email protected] Keywords: set-enrichment; ChemRICH; multi-omics; metabolic networks; candidate gene prioritization Received: March 12, 2019 Accepted: May 13, 2019 Published: June 11, 2019 Copyright: Barupal et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Estrogen-receptor negative (ERneg) breast cancer is an aggressive breast cancer subtype in the need for new therapeutic options. We have analyzed metabolomics, proteomics and transcriptomics data for a cohort of 276 breast tumors (MetaCancer study) and nine public transcriptomics datasets using univariate statistics, meta- analysis, Reactome pathway analysis, biochemical network mapping and text mining of metabolic genes. In the MetaCancer cohort, a total of 29% metabolites, 21% proteins and 33% transcripts were significantly different (raw p <0.05) between ERneg and ERpos breast tumors. -

Fine Mapping and Expression of Candidate Genes Within the Chromosome 10 QTL Region of the High and Low Alcohol Drinking Rats
View metadata, citation and similar papers at core.ac.uk brought to you by CORE HHS Public Access provided by IUPUIScholarWorks Author manuscript Author ManuscriptAuthor Manuscript Author Alcohol Manuscript Author . Author manuscript; Manuscript Author available in PMC 2017 July 18. Published in final edited form as: Alcohol. 2010 September ; 44(6): 477–485. doi:10.1016/j.alcohol.2010.06.004. Fine mapping and expression of candidate genes within the Chromosome 10 QTL region of the High and Low Alcohol Drinking Rats Paula J. Bice1, Tiebing Liang1, Lili Zhang1, Tamara J. Graves1, Lucinda G. Carr1, Dongbing Lai2, Mark W. Kimpel3, and Tatiana Foroud2 1Department of Medicine, Indiana University School of Medicine, Indianapolis, Indiana 46202, USA 2Department of Medical and Molecular Genetics, Indiana University School of Medicine, Indianapolis, IN 46202, USA 3Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN 46202, USA Abstract The high and low alcohol-drinking (HAD and LAD) rats were selectively bred for differences in alcohol intake. The HAD/LAD rats originated from the N/Nih heterogeneous stock (HS) developed from intercrossing 8 inbred rat strains. The HAD × LAD F2 were genotyped, and a powerful analytical approach, utilizing ancestral recombination as well as F2 recombination, was used to narrow a QTL for alcohol drinking to a 2 cM region on distal chromosome 10 that was in common in the HAD1/LAD1 and HAD2/LAD2 analyses. Quantitative real time PCR (qRT-PCR) was used to examine mRNA expression of 6 candidate genes (Crebbp, Trap1, Gnptg, Clcn7, Fahd1, and Mapk8ip3) located within the narrowed QTL region in the HAD1/LAD1 rats. -

Computational Analyses of Small Molecules Activity from Phenotypic Screens
Computational analyses of small molecules activity from phenotypic screens Azedine Zoufir Hughes Hall This dissertation is submitted for the degree of Doctor of Philosophy July 2018 Declaration This thesis is submitted as the result of my own work and includes nothing which is the outcome of work done in collaboration except where specifically indicated in the text. It is not substantially the same as any that I have submitted, or, is being concurrently submitted for a degree or diploma or other qualification at the University of Cambridge or any other University or similar institution except as declared in the preface and specified in the text. I further state that no substantial part of my dissertation has already been submitted, or, is being concurrently submitted for any such degree, diploma or other qualification at the University of Cambridge or any other University or similar institution except as declared in the Preface and specified in the text. This dissertation does not exceed the word limit of 60,000 words. Azedine Zoufir July 2018 Summary Title: Computational analyses of small molecules activity from phenotypic screens Author: Azedine Zoufir Drug discovery is no longer relying on the one gene-one disease paradigm nor on target-based screening alone to discover new drugs. Phenotypic-based screening is regaining momentum to discover new compounds since those assays provide an environment closer to the physiological state of the disease and allow to better anticipate off-target effects and other factors that can limit the efficacy of the drugs. However, uncovering the mechanism of action of the compounds active in those assays relies on in vitro techniques that are expensive and time- consuming. -

Prioritization of Metabolic Genes As Novel Therapeutic Targets
bioRxiv preprint doi: https://doi.org/10.1101/515403; this version posted January 9, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. 1 Prioritization of metabolic genes as novel therapeutic targets 2 in estrogen-receptor negative breast tumors using multi-omics data and text mining 3 4 Dinesh Kumar Barupal#,1, Bei Gao#, 1, Jan Budczies2, Brett S. Phinney4, Bertrand Perroud4, 5 Carsten Denkert2,3 and Oliver Fiehn*,1 6 Affiliations 7 1West Coast Metabolomics Center, University of California, Davis, CA, 95616 8 2Institute of Pathology, Charité University Hospital, Berlin, 9 3German Institute of Pathology, Philipps-University Marburg, Germany 10 4UC Davis Genome Center, University of California, Davis, CA, 95616 11 #Co-first authors and contributed equally. 12 Email address for all authors 13 Dinesh Kumar Barupal: [email protected] 14 Bei Gao: [email protected] 15 Carsten Denkert: [email protected] 16 Brett S. Phinney: [email protected] 17 Bertrand Perroud: [email protected] 18 Jan Budczies: [email protected] 19 Oliver Fiehn: [email protected] 20 21 * Corresponding Author: 22 Oliver Fiehn 23 West Coast Metabolomics Center, 24 University of California, Davis, CA, 95616 25 Email: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/515403; this version posted January 9, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Novel Non-Phosphorylative Pathway of Pentose Metabolism from Bacteria
www.nature.com/scientificreports OPEN Novel non-phosphorylative pathway of pentose metabolism from bacteria Received: 22 March 2018 Seiya Watanabe1,2,3, Fumiyasu Fukumori4, Hisashi Nishiwaki1,2, Yasuhiro Sakurai5, Accepted: 30 September 2018 Kunihiko Tajima5 & Yasuo Watanabe1,2 Published: xx xx xxxx Pentoses, including D-xylose, L-arabinose, and D-arabinose, are generally phosphorylated to D-xylulose 5-phosphate in bacteria and fungi. However, in non-phosphorylative pathways analogous to the Entner-Dodorof pathway in bacteria and archaea, such pentoses can be converted to pyruvate and glycolaldehyde (Route I) or α-ketoglutarate (Route II) via a 2-keto-3-deoxypentonate (KDP) intermediate. Putative gene clusters related to these metabolic pathways were identifed on the genome of Herbaspirillum huttiense IAM 15032 using a bioinformatic analysis. The biochemical characterization of C785_RS13685, one of the components encoded to D-arabinonate dehydratase, difered from the known acid-sugar dehydratases. The biochemical characterization of the remaining components and a genetic expression analysis revealed that D- and L-KDP were converted not only to α-ketoglutarate, but also pyruvate and glycolate through the participation of dehydrogenase and hydrolase (Route III). Further analyses revealed that the Route II pathway of D-arabinose metabolism was not evolutionally related to the analogous pathway from archaea. Te breakdown of D-glucose is central for energy and biosynthetic metabolism throughout all domains of life. Te most common glycolytic routes in bacteria are the Embden-Meyerhof-Parnas, the Entner-Doudorof (ED), and the oxidative pentose phosphate pathways. Te distinguishing diference between the two former glyco- lytic pathways lies in the nature of the 6-carbon metabolic intermediates that serve as substrates for aldol cleav- age. -

A Study of Alterations in DNA Epigenetic Modifications (5Mc and 5Hmc) and Gene Expression Influenced by Simulated Microgravity I
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Digital Repository @ Iowa State University Genome Informatics Facility Publications Genome Informatics Facility 1-28-2016 A Study of Alterations in DNA Epigenetic Modifications (5mC and 5hmC) and Gene Expression Influenced by Simulated Microgravity in Human Lymphoblastoid Cells Basudev Chowdhury Purdue University Arun S. Seetharam Iowa State University, [email protected] Zhiping Wang Indiana University School of Medicine Yunlong Liu Indiana University School of Medicine Amy C. Lossie Purdue University See next page for additional authors Follow this and additional works at: https://lib.dr.iastate.edu/genomeinformatics_pubs Part of the Bioinformatics Commons, Genetics Commons, and the Genomics Commons Recommended Citation Chowdhury, Basudev; Seetharam, Arun S.; Wang, Zhiping; Liu, Yunlong; Lossie, Amy C.; Thimmapuram, Jyothi; and Irudayaraj, Joseph, "A Study of Alterations in DNA Epigenetic Modifications (5mC and 5hmC) and Gene Expression Influenced by Simulated Microgravity in Human Lymphoblastoid Cells" (2016). Genome Informatics Facility Publications. 4. https://lib.dr.iastate.edu/genomeinformatics_pubs/4 This Article is brought to you for free and open access by the Genome Informatics Facility at Iowa State University Digital Repository. It has been accepted for inclusion in Genome Informatics Facility Publications by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. A Study of Alterations in DNA Epigenetic Modifications (5mC and 5hmC) and Gene Expression Influenced by Simulated Microgravity in Human Lymphoblastoid Cells Abstract Cells alter their gene expression in response to exposure to various environmental changes. Epigenetic mechanisms such as DNA methylation are believed to regulate the alterations in gene expression patterns. -

A Decade of Epigenetic Change in Aging Twins: Genetic and Environmental Contributions To
bioRxiv preprint doi: https://doi.org/10.1101/778555. this version posted June 12, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 A decade of epigenetic change in aging twins: genetic and environmental contributions to longitudinal DNA methylation Chandra A. Reynolds [1]*, Qihua Tan [2], Elizabeth Munoz [1]a, Juulia Jylhävä [3], Jacob Hjelmborg [2], Lene Christiansen [2,4], Sara Hägg [3], and Nancy L. Pedersen [3] [1] University of California, Riverside, USA [2] University of Southern Denmark, Odense, Denmark [3] Karolinska Institutet, Stockholm, Sweden [4] Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark a now at University of Texas at Austin, USA * Corresponding author: Chandra A. Reynolds, Department of Psychology, University of California Riverside, 900 University Avenue, Riverside, CA, 92521 USA; 951-827-2430 (tel); [email protected] bioRxiv preprint doi: https://doi.org/10.1101/778555. this version posted June 12, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 2 Summary/Abstract Background. Epigenetic changes may result from the interplay of environmental exposures and genetic influences and contribute to differences in age-related disease, disability and mortality risk. However, the etiologies contributing to stability and change in DNA methylation have rarely been examined longitudinally. Methods. We considered DNA methylation in whole blood leukocyte DNA across a 10-year span in two samples of same-sex aging twins: (a) Swedish Adoption Twin Study of Aging (SATSA; N = 53 pairs, 53% female; 62.9 and 72.5 years, SD=7.2 years); (b) Longitudinal Study of Aging Danish Twins (LSADT; N = 43 pairs, 72% female, 76.2 and 86.1 years, SD=1.8 years). -

Systems Genetics in Polygenic Autoimmune Diseases
From the Institute of Experimental Dermatology Director: Prof. Saleh Ibrahim Systems genetics in polygenic autoimmune diseases Dissertation for Fulfillment of Requirements for the Doctoral Degree of the University of Lübeck from the Department of Medicine Submitted by Yask Gupta from New-Delhi, India Lübeck 2015 i First referee:- Prof. Dr. med Saleh Ibrahim Second referee:- Prof. Dr. rer. nat. Jeanette Erdman Chairman:- Prof. Dr. Horst-Werner Stüzbecher Data of oral examination:- 7 June 2016 ii iii Contents 1. Introduction 9 1.1 Autoimmune diseases 3 1.2 Autoimmune bullous diseases 4 1.3 Epidermolysis bullosa acquisita (EBA): an autoantibody-mediated autoimmune skin disease 5 1.4 Role of miRNA in autoimmune diseases 5 1.5 Genes in autoimmune diseases 7 1.6 Gene networks 8 1.6.1 Gene/Protein interaction database 9 1.6.2 Ab intio prediction of gene networks 11 1.7 QTL and expression QTL 12 1.8. Prediction of ncRNA 13 1.8.1 Support vector machine and random forest 15 1.9. Structure of the thesis 18 2. Aims of the thesis 21 3. Materials and methods 23 3.1 Generation of a four way advanced intercross line 23 iv 3.2 Experimental EBA and observation protocol 24 3.2.1 Generation of recombinant peptides 24 3.3 Extraction of genomic DNA for genotypic analysis 25 3.4 Generation of microarray data (miRNAs/genes) and bioinformatics analysis 25 3.5 Software for Expression QTL analysis 26 3.5.1 HAPPY 27 3.5.2 EMMA 28 3.5.3 Implementation of software for expression QTL mapping 30 3.6 Ab initio Gene and microRNAs networks prediction 31 3.6.1 MicroRNAs 32 3.6.2 Genes 33 3.6.3 MicroRNAs gene target prediction 34 3.7 Visualization of interaction networks 35 3.8 Network databases (STRING and IPA) 36 3.9 Prediction of ncRNA tools 37 3.9.1 Dataset 38 3.9.2 Features for classification 39 3.9.3 Classification system 41 v 3.9.4 Implementation of support vector machines and random forest on the post transcriptional non coding RNA dataset 42 3.9.5 Work flow and output of ptRNApred 45 4. -

Setd5 Is Essential for Mammalian Development and the Co-Transcriptional Regulation of Histone Acetylation Anna B
© 2016. Published by The Company of Biologists Ltd | Development (2016) 143, 4595-4607 doi:10.1242/dev.141465 STEM CELLS AND REGENERATION RESEARCH ARTICLE Setd5 is essential for mammalian development and the co-transcriptional regulation of histone acetylation Anna B. Osipovich1,2, Rama Gangula1, Pedro G. Vianna1 and Mark A. Magnuson1,2,* ABSTRACT H3 and H4 has long been associated with transcriptionally active SET domain-containing proteins play a vital role in regulating genes and open chromatin, more recent studies have established that gene expression during development through modifications in histone methylations are also associated with actively transcribed chromatin structure. Here we show that SET domain-containing genes, including histone H3 methylation at lysines 4, 36 and 79 5(Setd5) is divergently transcribed with Gt(ROSA26)Sor,is (H3K4me, H3K36me and H3K79me) and histone H4 methylation necessary for mammalian development, and interacts with the at lysine 20 (H4K20me) (Smolle and Workman, 2013). Studies in PAF1 co-transcriptional complex and other proteins. Setd5-deficient yeast have shown that these histone modifications occur co- mouse embryos exhibit severe defects in neural tube formation, transcriptionally and require coordination between RNAP II, somitogenesis and cardiac development, have aberrant elongation complexes and regulators of histone modification vasculogenesis in embryos, yolk sacs and placentas, and die (Tanny, 2014). between embryonic day 10.5 and 11.5. Setd5-deficient embryonic Polymerase-associated factor 1 complex (PAF1C), which stem cells have impaired cellular proliferation, increased apoptosis, contains Paf1, Ctr9, Leo1, Cdc73 and Rtf1 as core components, defective cell cycle progression, a diminished ability to differentiate colocalizes to coding regions of genes and is involved in multiple into cardiomyocytes and greatly perturbed gene expression. -
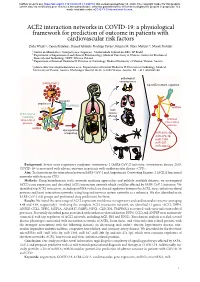
ACE2 Interaction Networks in COVID-19
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.13.094714; this version posted May 14, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. ACE2 interaction networks in COVID-19: a physiological framework for prediction of outcome in patients with cardiovascular risk factors Zofia Wicik1,2, Ceren Eyileten2, Daniel Jakubik2, Rodrigo Pavão1, Jolanta M. Siller-Matula2,3*, Marek Postula2 ¹ Centro de Matemática, Computação e Cognição - Universidade Federal do ABC, SP, Brazil ² Department of Experimental and Clinical Pharmacology, Medical University of Warsaw, Center for Preclinical Research and Technology CEPT, Warsaw, Poland ³ Department of Internal Medicine II, Division of Cardiology, Medical University of Vienna, Vienna, Austria * [email protected]; Department of Internal Medicine II, Division of Cardiology, Medical University of Vienna, Austria. Waehringer Guertel 18-20, A-1090 Vienna, Austria. Tel. +43 1 4040046140 pathological consequences SARS-CoV-2 top ACE2 network regulators activation attachment TMPRSS2 miRNA ACE2 main ACE2 network tissues containing affected virus-related activated proteins virus-related proteins miRNA dysregulation of signaling miR-302c-5p miR-27a-3p miR-1305 miR-587 miR-26b-5p Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (coronavirus disease 2019; COVID-19) is associated with adverse outcome in patients with cardiovascular disease (CVD). Aim: To characterize the interaction between SARS-CoV-2 and Angiotensin Converting Enzyme 2 (ACE2) functional networks with focus on CVD.