Prevalence and Antimicrobial Susceptibility Profile of Staphylococcus Aureus in Milk and Traditionally Processed Dairy Products in Addis Ababa, Ethiopia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FOREWORD Statistical Data That Reflect the Socio-Economic And
FOREWORD Statistical data that reflect the socio-economic and demographic conditions of the residents of a country are useful for designing and preparation of development plans as well as for monitoring and evaluation of the impact of the implementation of the development plans. These statistical data include population size, age, sex, literacy and education, marital status, housing stocks and conditions …etc. In order to fill the gap for these socio-economic and demographic data need, Ethiopia has conducted its third Population and Housing Census in the months of May and November 2007. The 2007 Population and Housing Census of Ethiopia was conducted under the auspices of the Population Census Commission that was established by a proclamation No. 449/1997. The Commission is chaired by the Deputy Prime Minister and the members of the commission were drawn from various relevant Ministries, Regional state representatives, House of Federation, National Electoral Board and the Central Statistical Agency, serving as the Office of the Census Commission (Secretariat). According to the proclamation, the processing, evaluation and analyses of the data collected in this census as well as its dissemination are the major responsibilities of this office. Thus, the Office of the Population Census Commission is pleased to present the census report entitled “The 2007 Population and Housing Census of Ethiopia: Statistical Report for Addis Ababa City Administration” for the users. Subsequently, another report which deals with detailed analysis of the result of the 2007 Population and Housing Census data is in the process of being prepared and will also be prepared and printed soon. Before the conduct of a census enumeration, numbers of preparatory activities were also carried-out. -

Addis Ababa City Structure Plan
Addis Ababa City Structure Plan DRAFT FINAL SUMMARY REPORT (2017-2027) AACPPO Table of Content Part I Introduction 1-31 1.1 The Addis Ababa City Development Plan (2002-2012) in Retrospect 2 1.2 The National Urban System 1.2 .1 The State of Urbanization and Urban System 4 1.2 .2 The Proposed National Urban System 6 1.3 The New Planning Approach 1.3.1 The Planning Framework 10 1.3.2 The Planning Organization 11 1.3.3 The Legal framework 14 1.4 Governance and Finance 1.4.1 Governance 17 1.4.2 Urban Governance Options and Models 19 1.4.3 Proposal 22 1.4.4 Finance 24 Part II The Structure Plan 32-207 1. Land Use 1.1 Existing Land Use 33 1.2 The Concept 36 1.3 The Proposal 42 2. Centres 2.1 Existing Situation 50 2.2 Hierarchical Organization of Centres 55 2.3 Major Premises and Principles 57 2.4 Proposals 59 2.5 Local development Plans for centres 73 3. Transport and the Road Network 3.1 Existing Situation 79 3.2 New Paradigm for Streets and Mobility 87 3.3 Proposals 89 4. Social Services 4.1 Existing Situation 99 4.2 Major Principles 101 4.3 Proposals 102 i 5. Municipal Services 5.1 Existing Situation 105 5.2 Main Principles and Considerations 107 5.3 Proposals 107 6. Housing 6.1 Housing Demand 110 6.2 Guiding Principles, Goals and Strategies 111 6.3 Housing Typologies and Land Requirement 118 6.4 Housing Finance 120 6.5 Microeconomic Implications 121 6.6 Institutional Arrangement and Regulatory Intervention 122 6.7 Phasing 122 7. -

IDENTIFYING MAJOR URBAN ROAD TRAFFIC ACCIDENT BLACK-SPOTS (Rtabss): a SUB-CITY BASED ANALYSIS of EVIDENCES from the CITY of ADDIS ABABA, ETHIOPIA
Journal of Sustainable Development in Africa (Volume 15, No.2, 2013) ISSN: 1520-5509 Clarion University of Pennsylvania, Clarion, Pennsylvania IDENTIFYING MAJOR URBAN ROAD TRAFFIC ACCIDENT BLACK-SPOTS (RTABSs): A SUB-CITY BASED ANALYSIS OF EVIDENCES FROM THE CITY OF ADDIS ABABA, ETHIOPIA Guyu Ferede Daie Addis Ababa University (AAU), Ethiopia ABSTRACT Despite its adverse socio-economic impact, a study on identification of road traffic accident black-spots (RTABSs) in Addis Ababa is either negligible or only a general attempt made for the city as a whole (National Road Safety Coordination Office, [NRSCO], 2005) without considering the specific experiences of each sub-city. The main aim of this study was, therefore, to identify the major accident black spots in each sub-city of Addis Ababa separately. For this purpose, secondary data obtained from Addis Ababa traffic police office was employed. The findings of the study revealed that there were 125 major accident black-spots in Addis Ababa as a whole. The distribution by sub-city shows10, 11, 24, 10, 21, 10, 20, 6, 4 and 9 RTABSs in Kirkos, Bole, Arada , Yeka, Lideta, Nifas-Silk/Lafto, Addis-Ketema, Akaki, Kolfe and Gullele sub-cities respectively. This has implication on the need for sustainable transport development planning. The RTABSs identified in each sub-city need to be focused while planning. Therefore, concerned bodies should encourage further investigation of specific causes for designing and implementing appropriate road safety control strategies in order to significantly reduce the incidence of road crashes in the city. This can be done by planning sustainable ways of designing transport system such as road networks that should accommodate the ever increasing number of vehicles followed by frequent inspection of vehicles themselves. -

Land Use Planning Strategies and Their Impacts on Public Transportation Supply of Addis Ababa (Mesalemiya- Kolfe-Aserasement Mazoriya-Lekuwanda Area)
Land use Planning Strategies and their Impacts on Public Transportation Supply of Addis Ababa (Mesalemiya- Kolfe-Aserasement Mazoriya-Lekuwanda Area) By: Henock Berhane Advisor: Alemayehu Ambo (Ph.D.) A Thesis Submitted to Graduate Studies, Addis Ababa Science & Technology University, College of Civil Engineering & Architecture, in Partial Fulfillment for the Requirements of the Degree of Masters of Science in Road and Transportation Engineering May, 2017 Addis Ababa Land use Planning Strategies and their Impacts on Public Transportation Supply of Addis Ababa (Mesalemiya-Kolfe-Aserasement Mazoria-Lekuwanda Area) By Henock Berhane Aberra APPROVED BY BOARD OF EXAMINERS ________________________________ ______________ _______________ Chairman, Department Graduate Committee Signature Date Dr. Alemayehu Ambo (Ph.D.) ______________ _______________ Advisor Signature Date Dr. Biruk Abate (PhD) _______________ _________________ Internal Examiner Signature Date Dr. Bikila Tiklu (PhD) _______________ __________________ External Examiner Signature Date Mr. Addisu B. _______________ __________________ (Head,Department of Civil Engineering) Signature Date Dr. Biruk Abate (PhD) _______________ _________________ (Dean, Architecture and Civil Engineering) Signature Date Statement of Certification This is to certify that Henock Berhane has carried out his research work on the topic entitled “Land use Planning Strategies and their Impacts on Public Transportation Supply of Addis Ababa (Mesalemiya-Kolfe-Aserasement Mazoriya-Lekuwanda Area)”. The work is original -

The Status of Green Spaces and Green Space Participatory Management in the City of Addis Ababa
Msc Programme in Urban Management and Development Rotterdam, The Netherlands September 2014 Thesis Title: The Status of Green spaces and Green space Participatory Management in the City of Addis Ababa Name: Gezahegne Gebremeskel Supervisor: Somesh Sharma Specialization: Urban Environmental Management UMD 10 MASTER’S PROGRAMME IN URBAN MANAGEMENT AND DEVELOPMENT (October 2013 – September 2014) Name: Gezahegne Gebremeskel Country: Ethiopia Supervisor: Somesh Sharma UMD 10 Report number: Rotterdam, September 2014 ii Summary The unprecedented rapid urbanization of Addis Ababa, coupled the city’s high population growth has been entailing intricacies on city’s management, amongst which Green spaces management constitute a part. In pursuit of the brown agenda i.e. infrastructural development, expansion of construction for settlement to accommodate the ever growing population, utilization of fuel wood and charcoal as bio fuel have been contributing to green spaces depletion in Addis. Green spaces participatory management has been a problem. This requires studies to find solution for the Green space management problem. This research’s aim is to determine the green spaces status and green spaces participatory management that entails stakeholder organizations and the community in the ten sub-cities of Addis Ababa. The main research methods are Land cover change detection using remotely sensed satellite multi-temporal raster data image analysis, qualitative and quantitative data analysis. The unit of analysis of this case study is organization. The main technical approach applied was qualitative data analysis acquired through purposive (judgmental) sampled data later analyzed using Atlas ti. To supplement the study, a quantitative sampled single point in time (2014) cross-sectional surveyed data of 100 respondents from the ten sub-cities of Addis was analyzed using SPSS. -

Terms of Reference
Terms of Reference GENERAL INFORMAION Services/Work Description: National Consultant for Serving as Lead Economist for Resettlement Action Plan (RAP) preparation for Beautifying Sheger Project (BSP) affected Households and Businesses along the major rivers in Addis Ababa Project Title: Beautifying Sheger Project-a programmatic approach to beautifying Sheger through sustainable urban resilience Post Title: Individual National Consultant (INC) Consultant Level: Level C – lead economist (Senior Specialist) Duty Station: Addis Ababa / Mega Projects Construction Office Duration: 30 calendar days Expected Start Date: Immediately after Signing the Contract I. BACKGROUND AND PROJECT CONTEXT Addis Ababa has been characterized by high population growth with over 5 million residents mainly due to rural-urban migration as youths and women migrate to the city to search for better livelihood opportunities. This high population growth is one of the causes for economic, social and environmental problems in the city. Economic related problems include high urban unemployment mainly among youths and women, increasing costs of living as demand for goods and services exceeds supply; fiscal problems (where Addis Ababa like other cities in developing countries lack enough funds/budget to pay for basic services such as housing, transport, policing, public education, wastes removal and street maintenance) and shortage of basic food and non-food items. Social related problems include crimes, traffic congestions, living in urban slums with overcrowded and lack pure drinking water, health facilities, toilet services, etc. In order to curb some of the social, economic and environmental problems mainly along river basins in the city where people are living in overcrowded urban slums, the Government of Ethiopia has launched a five- year project (2020 – 2021) – Beautifying Sheger Project (BSP)– to beautify banks of two major rivers with the combined distance of about 56 km running from Mount Entoto down to the bottom of the Akaki areas passing through the heavily populated part of the city. -

Urban Quality of Life and Its Spatial Distribution in Addis Ababa: Kirkos Sub-City
Urban Quality of Life and Its Spatial Distribution in Addis Ababa: Kirkos sub-city Elsa Sereke Tesfazghi March, 2009 Urban Quality of Life and Its Spatial Distribution In Addis Ababa: Kirkos sub-city By Elsa Sereke Tesfazghi Thesis submitted to the International Institute for Geo-information Science and Earth Observation in partial fulfilment of the requirements for the degree of Master of Science in Geo-information Science and Earth Observation, Specialisation: (Urban Planning and Management) Thesis Assessment Board Prof.Dr.Ir. M.F.A.M. van Maarseveen (Chairman) Dr. Karin Pfeffer (External Examiner) Dr. J.A. Martinez (First Supervisor) Drs J.J. Verplanke (Second Supervisor) INTERNATIONAL INSTITUTE FOR GEO-INFORMATION SCIENCE AND EARTH OBSERVATION ENSCHEDE, THE NETHERLANDS Disclaimer This document describes work undertaken as part of a programme of study at the International Institute for Geo-information Science and Earth Observation. All views and opinions expressed therein remain the sole responsibility of the author, and do not necessarily represent those of the institute. Dedicated to my late mother Tekea Gebru and my late sister Zufan Sereke Abstract Urban quality of life (QoL) is becoming the subject of urban research mainly in western and Asian countries. Such attention is due to an increasing awareness of the contribution of QoL studies in identifying problem areas and in monitoring urban planning policies. However, most studies are carried out at city or country level that commonly average out details at small scales. The result is that the variability of QoL at small scales is not well known. In addition, the relationship between subjective and objective QoL is not well known. -

Case of Addis Ababa (Nifas Silk Lafto Sub City)
ADDIS ABABA UNIVERSITY Institute Of Technology An Integrated Approach to Water Supply Planning: Case of Addis Ababa (Nifas Silk Lafto sub city) A Thesis Submitted to the School of Civil and Environmental Engineering Presented in Partial Fulfillment of the Requirements for the Degree of Master of Science (Water Supply and Environmental Engineering) By: Amsal Eyassu Dalle Advisor: Mebrate T. (Dr) October 2016 An Integrated Approach to Water Supply Planning: Case of Addis Ababa (Nifas Silk Lafto sub city) _____________________________________________________________________ ABSTRACT In planning and designing a water supply planning system, the key emphasis for meeting the growing water demand in Addis Ababa and elsewhere in the world is often focused on the endless desire to search for new resources and development of them. Limited effort is devoted to addressing in the issue in an integrated approach which combines the supply and demand side in a sustainable approach. Integrated water supply planning is a significant issue for sustainable development to maximize economy, secure production and minimize environmental impacts. The identification of sustainable water demand management practices is technically challenging because of lack of educated staff and attitude. Over the past few years, however, some efforts were seen in the developed countries to frame the water supply planning issue in an integrated fashion. However, this approach is not being practiced in developing countries, like Ethiopia, where the water supply problem is critical and the effort seems to repeat the track developed countries have taken without making the most out of the late comer position they maintain. With limited availability of water as a resource, the population pressure, the limited financial resource and other socio economic factors the traditional approach of endless investment is limited in its make to be a sustainable solution to water supply problems. -

St.Mary's University School of Graduate Studies Right of Way Acquisition and Its Influence on Road Construction Project Schedu
ST.MARY’S UNIVERSITY SCHOOL OF GRADUATE STUDIES RIGHT OF WAY ACQUISITION AND ITS INFLUENCE ON ROAD CONSTRUCTION PROJECT SCHEDULE PERFORMANCE THE CASE OF PROJECTS UNDER ADDIS ABABA ROAD AUTORITY BY AMANUAEL ALEMIE DESSIE (SGS/0391/2010A) JUNE, 2019 ADDIS ABABA, ETHIOPIA RIGHT OF WAY ACQUISITION AND ITS INFLUENCE ON ROAD CONSTRUCTION PROJECT SCHEDULE PERFORMANCE THE CASE OF PROJECTS UNDER ADDIS ABABA ROAD AUTORITY BY AMANUAEL ALEMIE DESSIE (SGS/0391/2010A) A THESIS SUBMITTED TO ST.MARY’S UNIVERSITY, SCHOOL OF GRATUATE STUDIES IN PATIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTERS OF ART IN PROJECT MANAGMENT JUNE, 2019 ADDIS ABABA, ETHIOPIA i ST.MARY’S UNIVERSITY SCHOOL OF GRADUATE STUDIES RIGHT OF WAY ACQUISITION AND ITS INFLUENCE ON ROAD CONSTRUCTION PROJECT SCHEDULE PERFORMANCE THE CASE OF PROJECTS UNDER ADDIS ABABA ROAD AUTORITY BY AMANUAEL ALEMIE DESSIE APPROVED BY BOARD OF EXAMINERS Dean, Graduate studies Signature & Date Advisor Signature & Date Internal Examiner Signature& Date External Examiner Signature& Date ii DECLARATION I, the undersigned, declared that this thesis is my original work, prepared under the guidance of Dereje Teklemariam (PhD). All sources of materials used for the thesis have been dually acknowledged. I further confirm that the thesis has not been submitted either in apart or in full to any other higher learning institution for the purpose of earning any degree. Name Signature St. Mary’s University, Addis Ababa June, 2019 iii ENDORCEMENT This thesis has been submitted to St. Mary’s university, school of graduate studies for examination with my approval as a university advisor. Advisor Signature St. -

City Profile Addis Ababa
SES Social Inclusion and Energy Managment for Informal Urban Settlements CITY PROFILE ADDIS ABABA Abnet Gezahegn Berhe, Dawit Benti Erena, Imam Mahmoud Hassen, Tsion Lemma Mamaru, Yonas Alemayehu Soressa CITY PROFILE ADDIS ABABA Abnet Gezahegn Berhe, Dawit Benti Erena, Imam Mahmoud Hassen, Tsion Lemma Mamaru, Yonas Alemayehu Soressa Funded by the Erasmus+ program of the European Union The European Commission support for the production of this publication does not constitute an endorsement of the contents which reflects the views only of the authors, and the Commission cannot be held responsible for any use which may be made of the information contained therein. The views expressed in this work and the accuracy of its findings is matters for the author and do not necessarily represent the views of or confer liability on the Centre of Urban Equity. © EiABC- Ethiopian Institute of Architecture Building Construction and City Development.. This work is made available under a Creative Commons Attribution 4.0 International Licence: https://creativecommons.org/licenses/by/4.0/ Contact: EiABC, P.O.Box 518, Addis Ababa www.eiabc.edu.et Suggested Reference: Erena D. et.al, (2017) City profile: Addis Ababa. Report prepared in the SES (Social Inclusion and Energy Management for Informal Urban Settlements) project, funded by the Erasmus+ Program of the European Union. http://moodle.donau-uni.ac.at/ses/ 2 CITY PROFILE ADDIS ABABA ABSTRACT Addis Ababa, the capital city of Ethiopia and the The reaction of the present government to these chal- diplomatic centre of Africa, embodies a 130 years lenges is expressed in its growth and transformation of development history that contributes to its cur- programme that embrace the urban development rent socio-spatial features. -
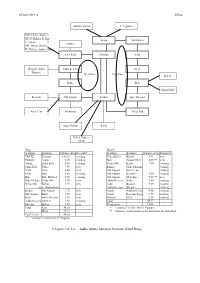
S.Figure 9.8-1-A Addis Ababa Junction Network (Dual Ring) S.Figure 9.8-1-A S.Ring
S.Figure 9.8-1-a D.Ring Addisu Gebeya F. Legation NW: Fitche, Kuyu NE: D.Birhan, D.Sina Arada Sidist Kilo S: Sebeta (MSC) SW: Ghion, Wolkite W: Holeta, Ambo, Guder AA TR-III Filwoha Yeka Shegole, Asko, Addis Ketema Gerji Burayu West Ring East Ring Kotebe Kolfe Bole Airport DLC Keranio Old Airport Kirkos Bole Michael Ayer Tena Mekanisa Nefas Silk Hana Mariam Kaliti D.Zeit, Dukem, Akaki Ring Branch A station B station Distance (km) Remarks* A station B station Distance (km) Remarks* TR/ITE Filwoha 1.6/2.0 existing Yeka (Bole) Kotebe5.84 new Filwoha Arada3.00 existing Bole Airport DLC4.00 ** new Arada Sidist Kilo5.20 existing Nefas Silk Kaliti7.40 existing Sidist Kilo Yeka7.90 new Kirkos Hana Mariam existing Yeka Gerji6.40 new Old Airport Ayer Tena existing Gerji Bole5.50 existing Old Airport Keranio5.50 existing Bole Bole Michael4.50 existing Old Airport Mekanisa5.00 ** new Bole Michael Nefas Silk3.70 new Addis Ketema Asko7.60 existing Nefas Silk Kirkos4.50 new Asko Burayu5.00 existing (route duplication) Addis Ketema Shegole existing Kirkos Old Airport3.72 new Arada Addisu Gebeya 4.80 existing Old Airport Kolfe7.20 new Arada Ferensay Lega 6.33 existing Kolfe Addis Ketema3.10 new Filwoha ECA2.80 existing Addis Ketema TR/ITE3.60 existing Total 54.27 Filwoha Kirkos3.50 new Total (new) 14.84 Total East44.20 * "existing" includes 8th D. Program West23.12 ** Distance is provisional as the location is not identified. Total (new) 40.02 * "existing" includes 8th D. Program S.Figure 9.8-1-a Addis Ababa Junction Network (Dual Ring) S.Figure 9.8-1-a S.Ring Addisu Gebeya F. -

Measuring Efficiency of Public Health Centers in Ethiopia
2016 Measuring Efficiency of Public Health Centers in Ethiopia Carlyn Mann, Ermias Dessie, Mideksa Adugna, and Peter Berman Resource Tracking and Management Project Primary Health Care Cost Study Series: Paper 3 December 2016 Measuring Efficiency of Public Health Centers in Ethiopia Acknowledgements The authors would like to thank many colleagues at the Federal Ministry of Health, Breakthrough International Consultancy, and Harvard T.H. Chan School of Public Health as well as Harvard Global Research and Support Services, Inc. for their valuable support and inputs for this report. The constant support from Dr. Mizan Kiros, Director of the Financial Resource Mobilization Directorate (FRM) of FMOH, Ato Mideksa Adugna from FRM, and other FRM staff was much needed for this work to come into fruition. We would also like to extend our thanks to key staff at FMOH that provided the guidance and leadership during the primary health care cost study that provided the much needed data to conduct the efficiency analysis this report covers. Of particular note are: Dr. Addis Tamire, Ato Abdujelil Reshad, Dr. Sinetsehay Chanayalew, and Dr. Mekdim Enkossa. Furthermore, Anubhav Agarwal conducted the data cleaning and cost accounting that made it possible to utilize the data from the PHC cost study. Sarah Hurlburt provided editorial support and design of this report. Grant support from the Bill & Melinda Gates Foundation as part of the Resource Tracking and Management (RTM) Project, a three year program, at the Harvard T.H. Chan School of Public Health in partnership with Breakthrough International Consultancy, PLC is gratefully acknowledged. All errors remain the responsibility of the authors.