Review Diverse Midbrain Dopaminergic Neuron Subtypes And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Safety of Influenza Vaccines in Children
Vaccine 33 (2015) F1–F67 Contents lists available at ScienceDirect Vaccine j ournal homepage: www.elsevier.com/locate/vaccine Review The safety of influenza vaccines in children: An Institute for Vaccine ଝ,ଝଝ Safety white paper a,b,∗ b,c d a Neal A. Halsey , Kawsar R. Talaat , Adena Greenbaum , Eric Mensah , a,b a,b a,b Matthew Z. Dudley , Tina Proveaux , Daniel A. Salmon a Department of International Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, United States b Institute for Vaccine Safety, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, United States c Center for Immunization Research, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States d Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, MD, United States a r t i c l e i n f o a b s t r a c t Keywords: Most influenza vaccines are generally safe, but influenza vaccines can cause rare serious adverse events. Influenza Some adverse events, such as fever and febrile seizures, are more common in children than adults. There Influenza vaccine can be differences in the safety of vaccines in different populations due to underlying differences in Vaccine safety genetic predisposition to the adverse event. Live attenuated vaccines have not been studied adequately Local reactions following IIV in children under 2 years of age to determine the risks of adverse events; more studies are needed to Cellulitis-like reactions address this and several other priority safety issues with all influenza vaccines in children. -

1 Before the U.S. COPYRIGHT OFFICE, LIBRARY of CONGRESS
Before the U.S. COPYRIGHT OFFICE, LIBRARY OF CONGRESS In the Matter of Exemption to Prohibition on Circumvention of Copyright Protection Systems for Access Control Technologies Under 17 U.S.C. §1201 Docket No. 2014-07 Reply Comments of the Electronic Frontier Foundation 1. Commenter Information Mitchell L. Stoltz Corynne McSherry Kit Walsh Electronic Frontier Foundation 815 Eddy St San Francisco, CA 94109 (415) 436-9333 [email protected] The Electronic Frontier Foundation (EFF) is a member-supported, nonprofit public interest organization devoted to maintaining the traditional balance that copyright law strikes between the interests of rightsholders and the interests of the public. Founded in 1990, EFF represents over 25,000 dues-paying members, including consumers, hobbyists, artists, writers, computer programmers, entrepreneurs, students, teachers, and researchers, who are united in their reliance on a balanced copyright system that ensures adequate incentives for creative work while promoting innovation, freedom of speech, and broad access to information in the digital age. In filing these reply comments, EFF represents the interests of the many people in the U.S. who have “jailbroken” their cellular phone handsets and other mobile computing devices—or would like to do so—in order to use lawfully obtained software of their own choosing, and to remove software from the devices. 2. Proposed Class 16: Jailbreaking – wireless telephone handsets Computer programs that enable mobile telephone handsets to execute lawfully obtained software, where circumvention is accomplished for the sole purposes of enabling interoperability of such software with computer programs on the device or removing software from the device. 1 3. -

Dean's List | Illinois Students
Univeristy of Illinois at Urbana-Champaign | Fall 2011 | Dean's List | Illinois Students Middle Student City ZIP Last Name First Name College Major Name Class Addison Adams Sarah Elizabeth 3 Agricultural, Consumer & Environmental Sciences Animal Sciences Addison Cruce Justin T 1 Engineering Civil Engineering Addison Foster Kayla E 1 Liberal Arts & Sciences Biology Addison Gerth Christopher M 2 Engineering Electrical Engineering Addison Kelly Rebecca R 1 Agricultural, Consumer & Environmental Sciences Food Science & Human Nutrition Addison Kwee Dustin J 1 Business Curric Unassigned Addison Patel Megh J 1 Liberal Arts & Sciences Molecular and Cellular Biology Addison Ramir Tyler J 2 Division of General Studies Undeclared Addison Ramirez Amanda Marie 4 Liberal Arts & Sciences Psychology Addison Rowley Jennifer Marie 2 Liberal Arts & Sciences English Addison Saporito Daniela Clara 4 Liberal Arts & Sciences Communication Algonquin Badmus Oyindamola Hanna Y2 Law Law Algonquin Blunk Melissa Kelly 3 Applied Health Sciences Recreation, Sport & Tourism Algonquin Demetriou Nicholas P 1 Engineering Materials Science & Engineering Algonquin Dobbelaere Andie V 2 Business Marketing Algonquin Dombrowski Anthony J 3 Fine & Applied Arts Architectural Studies Algonquin Gardeck Matthew John 4 Business Accountancy Algonquin Kaczar Brian C 1 Business Curric Unassigned Algonquin Kale Brett W 1 Business Curric Unassigned Algonquin Koniewicz Kristen L 2 Liberal Arts & Sciences Biology Algonquin Kunzweiler Christopher K 4 Business Accountancy Algonquin Lindgren -

Banking Act Unclaimed Money As at 31 December 2007
Commonwealth of Australia Gazette No. ASIC 40A/08, Wednesday, 21 May 2008 Published by ASIC ASIC Gazette Contents Banking Act Unclaimed Money as at 31 December 2007 RIGHTS OF REVIEW Persons affected by certain decisions made by ASIC under the Corporations Act 2001 and the other legislation administered by ASIC may have rights of review. ASIC has published Regulatory Guide 57 Notification of rights of review (RG57) and Information Sheet ASIC decisions – your rights (INFO 9) to assist you to determine whether you have a right of review. You can obtain a copy of these documents from the ASIC Digest, the ASIC website at www.asic.gov.au or from the Administrative Law Co-ordinator in the ASIC office with which you have been dealing. ISSN 1445-6060 (Online version) Available from www.asic.gov.au ISSN 1445-6079 (CD-ROM version) Email [email protected] © Commonwealth of Australia, 2008 This work is copyright. Apart from any use permitted under the Copyright Act 1968, all rights are reserved. Requests for authorisation to reproduce, publish or communicate this work should be made to: Gazette Publisher, Australian Securities and Investment Commission, GPO Box 9827, Melbourne Vic 3001 ASIC GAZETTE Commonwealth of Australia Gazette ASIC 40A/08, Wednesday, 21 May 2008 Banking Act Unclaimed Money Page 2 of 463 Specific disclaimer for Special Gazette relating to Banking Unclaimed Monies The information in this Gazette is provided by Authorised Deposit-taking Institutions to ASIC pursuant to the Banking Act (Commonwealth) 1959. The information is published by ASIC as supplied by the relevant Authorised Deposit-taking Institution and ASIC does not add to the information. -

Commencement May2021 Progr
CLASS OF COMMENCEMENT MAY 15, 2021 II The pandemic did not diminish your years of hard work, nor did it define who you will become. In fact, it has brought into sharp focus the remarkable humans you already are. Your ability to reach this day — GRADUATING FROM THE UNIVERSITY OF CALIFORNIA, BERKELEY — is nothing short of heroic. Congratulations, CLASS OF 2021. PROGRAM WELCOME AND LAND ACKNOWLEDGEMENT ALFRED DAY ASSOCIATE DEAN OF STUDENTS AND DIRECTOR CENTER FOR SUPPORT AND INTERVENTION NATIONAL ANTHEM JAE JOON (JOSEPH) SHIN ’21 INTRODUCTION OF CHANCELLOR T. CHRIST NIKITA DHAR ’21 VICE PRESIDENT, SENIOR CLASS COUNCIL WELCOME REMARKS AND AWARD PRESENTATION CAROL T. CHRIST CHANCELLOR THE UNIVERSITY MEDALIST LEYLA KABULI ’21 STUDENT PERFORMANCE OWEN FARMER ’21 GUINNESS WORLD RECORD UNICYCLIST CLASS OF 2021 SENIOR GIFT PRESENTATION DOUGLAS WICKHAM ’21 PRESIDENT, CAL STUDENT PHILANTHROPY ERIC MANZO ’21 VICE PRESIDENT OF MEMBERSHIP, CAL STUDENT PHILANTHROPY INTRODUCTION OF KEYNOTE SPEAKER MEGAN WIENER ’21 PRESIDENT, SENIOR CLASS COUNCIL KEYNOTE SPEAKER ADEWALE “WALLY” ADEYEMO ’04 DEPUTY SECRETARY OF THE TREASURY STUDENT PERFORMANCE BARETROUPE CONFERRING OF DEGREES CAROL T. CHRIST CHANCELLOR HAIL TO CALIFORNIA DECADENCE A CAPPELLA 2 KEYNOTE SPEAKER ADEWALE “WALLY” ADEYEMO ’04 Wally Adeyemo, the Deputy Secretary of the Treasury, has spent most of his career in public service, convening governments, companies, and organizations to achieve common goals. Adeyemo came to the Treasury Department from the Obama Foundation, where he served as president beginning in August 2019. Before that he was a senior advisor at the Center for Strategic and International Studies and at Blackrock and held several public service roles. -
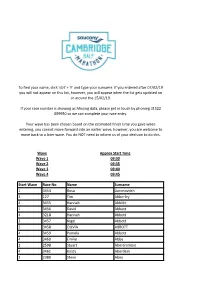
Start Wave Race No. Name Surname 1 3454 Rosa Aaronovitch 3 127 Tim
To find your name, click 'ctrl' + 'F' and type your surname. If you entered after 07/02/19 you will not appear on this list, however, you will appear when the list gets updated on or around the 15/02/19. If your race number is showing as Missing data, please get in touch by phoning 01522 699950 so we can complete your race entry. Your wave has been chosen based on the estimated finish time you gave when entering, you cannot move forward into an earlier wave, however, you are welcome to move back to a later wave. You do NOT need to inform us of your decision to do this. Wave Approx Start Time Wave 1 09:30 Wave 2 09:35 Wave 3 09:40 Wave 4 09:45 Start Wave Race No. Name Surname 1 3454 Rosa Aaronovitch 3 127 Tim Abberley 4 3455 Hannah Abblitt 1 3456 David Abbott 4 3218 Hannah Abbott 3 3457 Nigel Abbott 2 3458 OLIVIA ABBOTT 4 3459 Pamela Abbott 4 3460 Emilie Abby 2 2598 Stuart Abercrombie 4 3461 Kirsty Aberdein 2 2380 Steve Abey 3 3462 Teresa Ablewhite 3 3463 Alvin Abraham 3 154 Deborah Abraham 2 155 Brett Abram 1 156 Qasim Abul As 3 157 Annalisa Accascina 3 3022 Cosette Ackland 1 3464 Nathan Ackroyd 3 3465 Amanda Acott 2 3466 EVA ACS 3 3467 Anita Adam 2 3468 Rob Adam 1 3469 Benjamin Adams 1 3470 Charles Adams 4 3471 Christine Adams 2 158 Dan Adams 1 3472 David Adams 4 159 Isabel Adams 1 3473 Jonathan Adams 1 160 Matthew Adams 1 3474 Neil Adams 2 3475 Patrick Adams 1 3476 Paul Adams 2 161 Rebecca Adams 3 3477 sophie Adams 4 3478 Sophie Adams 1 3479 Tom Adams 4 3047 faron adamson 1 3480 John Adamson 1 3481 Jack Adcock 2 3482 Jon Adcock 3 3483 -

Surname First Name Hx Tree Mem Ories VF Bk Other Names & Misc. A
Surname First Name Hx Tree Mem VF Bk Other Names & Misc. ories A file Info for Robinson Anderson Adams Moses N X X Gaul Adolphson Andrew X X Herberg Ahlstrom Jacob X X Elmquist, Lund, Melin Aiton Mary X X Historical article Almen Carl X X X Person, Hedeen, Vickner Alwine Sebastian X X Young Anderson Anders X Census Anderson Charles X X X Swenson, Johnson, Carlson Anderson Christian X Excerpt of Hx of Mn Valley Anderson George X Diary from Civil War Anderson John/Delia X Armstrong - Opera family Anderson Magnus X X Jungbloom, Magnusson Anderson Marion X Artist - articls Anderson Mathilda X Confirmation certificate Anderson Peter/Martha X X X Andexstad Albert/Cathy X Family Foundation Annexstad Martin/Hattie C X Misc Info. Interview Annexstad Ole / Martha X X X X Schmidt, Humphrey, Arneman, Germscheid, Quist, Paquette, Goblirsch, Gilbertson, Ewing, Strand, Snyder, Estesen, Braun, Peterson Annexstad Ole/Marie X Tollefson, Quist, Overn Anthony Lois X X X X Dahl, Strand, Hanson, Struck Anton Frank / Mary X X X X Blumhoefer, Klingler, Binder, Fesenmaier, Grathwohl, Heinze, Hedwig Arnsdorf William/Catherine X X Klages, Gintner Austin Horace, Gov X Governor's Address see also McGill Baasen Francis X Lifetime achievements Baker Nathan X X Abbreviated Fam Tree Bang Olaf / Oline X X X X Lohre, Holz, Christenson, Nilsen, Rinkel, Stensrud, Hagen, Hawn, Harria, Gilbert, Majerus, Hilbert Barnes Newell Albert / Gertrude X X Descent Chart (Dean) Bartz Frederick X X X Vogt, Pagel, Schwartz, Groth, Kasten, Pehling, Kamholz, Oslund, Hayer, Seibert Bean Aaron X X Shields Becker Conrad X X X Dallmann, Voges, Harmening, Terhofter, Klippstein, Battig, Werth, Boelter, Liebl, Huelskamp, Wills, Ruble, Donnelly, Enter, Baumann, Weydert Behneke August X X Miscellaneous info re History Bell Samuel X X Turtle MHS Death Cert ID No. -

Greeks Helping Greeks
Greeks Helping Greeks ANNUAL REPORT 2019 About THI The Hellenic Initiative (THI) is a global, nonprofi t, secular institution mobilizing the Greek Diaspora and Philhellene community to support sustainable economic recovery and renewal for Greece and its people. Our programs address crisis relief through strong nonprofi t organizations, led by heroic Greeks that are serving their country. They also build capacity in a new generation of heroes, the business leaders and entrepreneurs with the skills and values to promote the long term growth of Hellas. THI Vision / Mission Statement Investing in the future of Greece through direct philanthropy and economic revitalization. We empower people to provide crisis relief, encourage entrepreneurs, and create jobs. We are The Hellenic Initiative (THI) – a global movement of the Greek Diaspora About the Cover Featuring the faces of our ReGeneration Interns. We, the members of the Executive Committee and the Board of Directors, wish to express to all of you, the supporters and friends of The Hellenic Initiative, our deepest gratitude for the trust and support you have given to our organization for the past seven years. Our mission is simple, to connect the Diaspora with Greece in ways which are valuable for Greece, and valuable for the Diaspora. One of the programs you will read about in this report is THI’s ReGeneration Program. In just 5 years since we launched ReGeneration, with the support of the Coca-Cola Co. and the Coca-Cola Foundation and 400 hiring partners, we have put over 1100 people to work in permanent well-paying jobs in Greece. -

Artículos Científicos
Editor: NOEL GONZÁLEZ GOTERA Número 122 Diseño: Lic. Roberto Chávez y Liuder Machado. Semana 080214– 140214 Fotos: Lic. Belkis Romeu e Instituto Finlay La Habana, Cuba. ARTÍCULOS CIENTÍFICOS Publicaciones incluidas en PubMED durante el período comprendido entre el 7 y el 14 de febrero de 2014. Con “vaccin*” en título: 119 artículos recuperados. Vacunas Meningococo (Neisseria meningitidis) 108. Review of meningococcal vaccines with updates on immunization in adults. Zahlanie YC, Hammadi MM, Ghanem ST, Dbaibo GS. Hum Vaccin Immunother. 2014 Feb 5;10(4). [Epub ahead of print] Review. PMID: 24500529 [PubMed - as supplied by publisher] Related citations Select item 24500396 Vacunas BCG – ONCO BCG (Mycobacterium bovis) 104. Pulmonary BCG vaccination confers dose-dependent superior protection than subcutaneous vaccination. Aguilo N, Toledo AM, Lopez-Roman EM, Perez-Herran E, Gormley E, Rullas-Trincado J, Angulo- Barturen I, Martin C. Clin Vaccine Immunol. 2014 Feb 5. [Epub ahead of print] PMID: 24501340 [PubMed - as supplied by publisher] Related citations Select item 24501164 1 Vacunas Neumococo (Streptococcus pneumoniae) 68. Clinical features of adults with seven-valent-conjugated-vaccine-serotype pneumococcal pneumonia. Rodrigo C, Bewick T, Sheppard C, Greenwood S, Trotter C, Slack M, George R, Lim WS. Vaccine. 2014 Feb 6. pii: S0264-410X(14)00067-X. doi: 10.1016/j.vaccine.2014.01.035. [Epub ahead of print] PMID: 24508039 [PubMed - as supplied by publisher] Related citations Select item 24508037 76. Modeling the impact of the 7-valent pneumococcal conjugate vaccine in Chinese infants: an economic analysis of a compulsory vaccination. Che D, Zhou H, He J, Wu B. BMC Health Serv Res. -

Recreational Diving and Its Effects on The
phy ra and og n M Siciliano et al., J Oceanogr Mar Res 2016, 4:2 a a r e i c n e O DOI: 10.4172/2572-3103.1000151 f R Journal of o e l s a e a n r r c ISSN:u 2572-3103 h o J Oceanography and Marine Research Research Article Open Access Recreational Diving and Its Effects on the Macroalgal Communities of the Unintentional Artificial Reef Zenobia Shipwreck (Cyprus) Siciliano A1*, Jimenez C2,3 and Petrou A2 1Marine Ecology Research Group, University of Canterbury, Christchurch, New Zealand 2Enalia Physis Environmental Research Centre, Aglantzia, Nicosia, Cyprus 3Energy, Environment and Water Research Center of the Cyprus Institute, Nicosia, Cyprus Abstract The ecological role of shipwrecks as artificial reefs is well established and often is prime and exclusive destinations for diving tourism. But they are also extremely delicate and sensitive environments. For this reason, the impact of recreational diving on shipwrecks should be taken in consideration since diver’s experience can strongly affect their associated benthic communities. The aim of this study was to verify the impact of anthropogenic activities (scuba divers) on the macroalgal coverage, here considered as indicator of physical disturbance, on the modern shipwreck Zenobia, in Cyprus (east Mediterranean Sea). Divers behaviour was investigated in the wreck and the macroalgal coverage was determined (photo-quadrat method) in three areas differently exposed to physical contact of divers. Our results suggest that diving is having a significant negative effect on the macroalgae coverage of the shipwreck, especially in areas subject to high levels of use (e.g., meeting stations) when compared to control sites in the same wreck. -

031--Rostrevor--Magazine-March-2010.Pdf
rostrevor1 A Catholic School in the Edmund Rice Tradition March 2010 ROSTREVOR CoLLeGe inside Palma Merenti Chair of the Board From the Headmaster Senior School Middle School Junior School Scholastic Highlights Red and Black Rostrevor’s Heritage – Handball Bob Taylor Peter Tunbridge Nerio Ferraro Mark Shadiac Christian Brother Jubilees ROCSC ROCFC QUARTERLY Print Post Approved PPP 535216-00029 Rostrevor College Glen Stuart Road, Woodforde South Australia 5072 Telephone 08 8364 8200 Facsimile 08 8364 8396 email [email protected] www.rostrevor.sa.edu.au 2 Palma Merenti From the Chairperson finally, the Victorian Government has heard and answered the call … even in the face of challenging economic times. In so doing, the Victorian Government has recognised: The right of parents and families to choose schools that they believe will best meet the needs of their children. The valuable contribution Catholic and Independent schools make in the provision of educational opportunity and the achievement of quality schooling. That parents and families have unfairly shouldered increasing responsibility for the costs that come with their decision Performing at the Presentation Night from Left: James Evenden, Ilario Spano and Alex Trevorrow to choose a Catholic or Independent school education for their children. The seriously under-whelming news is that, as a result, our state government Chair of the Board Report now invests less in Catholic and Delivered at Presentation Night Independent school students than any 24th November 2009 other State government in Australia - and, worse, the level of funding it is Last week, a newspaper headline providing is declining. shouted “State hands $2.1billion to non- Government funding of schools is a government schools” … but guess complex, emotional and hotly debated what? The newspaper wasn’t The topic. -

Magnetotransport in Cleaved-Edge-Overgrown F E/Gaas-Based and Rare-Earth-Doped Gan-Based Heterostructures
Magnetotransport in Cleaved-Edge-Overgrown F e/GaAs-based and Rare-Earth-Doped GaN-based Heterostructures Dissertation zur Erlangung des Grades eines Doktors der Naturwissenschaften in der Fakultat fur Physik und Astronomie der Ruhr-Universitat Bochum vorgelegt von Fang-Yuh Lo geboren in Taipei, Taiwan Lehrstuhl fur Angewandte Festkorperphysik 2007 Der erste Gutachter: Prof. Dr. Andreas D. Wieck Der zweite Gutachter: Prof. Dr. Daniel Hagele Date of Disputation: 05.07.2007 1 Table of contents Table of contents 1 List of abbreviations 3 List of symbols 5 1 Introduction 7 2 Theoretical background 11 2.1 Introduction to GaAs- and GaN-based heterostructures 11 2.2 Magnetism 14 2.2.1 Isolated atoms 14 2.2.2 Magnetism in materials 15 2.2.2.1 Exchange interactions 15 2.2.2.2 Molecular field theory and ab-initio calculations 16 2.2.2.3 Magnetic materials 18 2.3 Magnetotransport and electrical spin injection 20 2.3.1 Magnetoresistance in nonmagnetic materials 20 2.3.2 Magnetoresistance in ferromagnetic materials 22 2.3.3 Electrical spin injection 24 2.4 GaN-based diluted magnetic semiconductors 29 2.5 Focused ion beam 32 2.5.1 Focused ion beam system 33 2.5.1.1 Liquid metal ion source 33 2.5.1.2 Focused ion beam column 34 2.5.2 Focused ion beam milling 36 2.5.2.1 sputtering 36 2.5.2.2 Redeposition 37 2.5.2.3 Implantation and amorphization 38 2 3 Magnetotransport in cleaved-edge-overgrown Fe/GaAs-based heterostructures 39 3.1 Cleaved-edge overgrowth 39 3.1.1 Properties of the interface and the metal thin films 41 3.1.2 Properties of the metal-semiconductor