Transient Diabetes Insipidus in Pregnancy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gestational Diabetes Insipidus, HELLP Syndrome and Eclampsia in a Twin Pregnancy: a Case Report
Journal of Perinatology (2010) 30, 144–145 r 2010 Nature Publishing Group All rights reserved. 0743-8346/10 $32 www.nature.com/jp PERINATAL/NEONATAL CASE PRESENTATION Gestational diabetes insipidus, HELLP syndrome and eclampsia in a twin pregnancy: a case report JL Woelk, RA Dombroski and PR Brezina Department of Obstetrics and Gynecology, East Carolina University, Greenville, NC, USA alanine aminotransferase, 65 U lÀ1; and lactate dehydrogenase, We report a case of eclampsia in a twin pregnancy complicated by HELLP 390 U lÀ1. A 24-h urine collection was begun to quantify proteinuria. syndrome and diabetes insipidus. This confluence of disease processes During the first night of hospitalization, the patient developed suggests that a modification of common magnesium sulfate treatment marked polyuria and polydipsia. Urine output increased to 1500 cc hÀ1 protocols may be appropriate in a certain subset of patients. by the early morning totaling 12 l for the entire night. Repeat Journal of Perinatology (2010) 30, 144–145; doi:10.1038/jp.2009.115 electrolytes were unchanged. The endocrinology service was then Keywords: diabetes insipidus ; eclampsia ; HELLP syndrome ; twin consulted for the management of presumptive DI. Therapy with the pregnancy ; magnesium sulfate administration of dDAVP (1-deamino-8-D-arginine vasopressin) orally twice daily was initiated. Serum osmolality was increased at 296 mOsm kgÀ1, and urine osmolality was decreased to 71 mOsm kgÀ1 Introduction with a specific gravity of 1.000. The 24-h urine results showed a total The association between diabetes insipidus (DI) and liver protein of 780 mg. The patient denied the previously reported dysfunction in a preeclamptic patient has been established in headaches, blurry vision and right upper quadrant pain, and her blood multiple case reports.1 This case is an important example that pressures remained 140 to 155 mmHg (systolic) and 80 to 95 mmHg illustrates how the pathophysiology of DI and the pharmacokinetics (diastolic), consistent with a diagnosis of mild preeclampsia. -
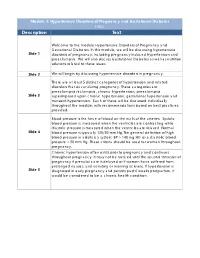
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text Welcome to the module Hypertensive Disorders of Pregnancy and Gestational Diabetes. In this module, we will be discussing hypertensive Slide 1 disorders of pregnancy, including pregnancy induced hypertension and preeclampsia. We will also discuss Gestational Diabetes as well as nutrition solutions related to these issues Slide 2 We will begin by discussing hypertensive disorders in pregnancy. There are at least 5 distinct categories of hypertension and related disorders that occur during pregnancy. These categories are: preeclampsia/eclampsia, chronic hypertension, preeclampsia Slide 3 superimposed upon chronic hypertension, gestational hypertension and transient hypertension. Each of these will be discussed individually throughout the module, with recommendations based on best practices provided. Blood pressure is the force of blood on the walls of the arteries. Systolic blood pressure is measured when the ventricles are contracting while diastolic pressure is measured when the ventricles are relaxed. Normal Slide 4 blood pressure is typically 120/80 mm Hg.The general definition of high blood pressure in adults is a systolic BP > 140 mg HG or a diastolic blood pressure > 90 mm Hg. These criteria should be used for women throughout pregnancy. Chronic hypertension often exists prior to pregnancy and continues throughout pregnancy. It may not be noticed until the second trimester of pregnancy if prenatal care is delayed or if women have suffered from prolonged nausea and vomiting or morning sickness. If hypertension is Slide 5 diagnosed in early pregnancy and persists past 6 weeks postpartum, it would be considered to be a chronic health condition. -

Role of Maternal Age and Pregnancy History in Risk of Miscarriage
RESEARCH Role of maternal age and pregnancy history in risk of BMJ: first published as 10.1136/bmj.l869 on 20 March 2019. Downloaded from miscarriage: prospective register based study Maria C Magnus,1,2,3 Allen J Wilcox,1,4 Nils-Halvdan Morken,1,5,6 Clarice R Weinberg,7 Siri E Håberg1 1Centre for Fertility and Health, ABSTRACT Miscarriage and other pregnancy complications might Norwegian Institute of Public OBJECTIVES share underlying causes, which could be biological Health, PO Box 222 Skøyen, To estimate the burden of miscarriage in the conditions or unmeasured common risk factors. N-0213 Oslo, Norway Norwegian population and to evaluate the 2MRC Integrative Epidemiology associations with maternal age and pregnancy history. Unit at the University of Bristol, Introduction Bristol, UK DESIGN 3 Miscarriage is a common outcome of pregnancy, Department of Population Prospective register based study. Health Sciences, Bristol Medical with most studies reporting 12% to 15% loss among School, Bristol, UK SETTING recognised pregnancies by 20 weeks of gestation.1-4 4Epidemiology Branch, National Medical Birth Register of Norway, the Norwegian Quantifying the full burden of miscarriage is Institute of Environmental Patient Register, and the induced abortion register. challenging because rates of pregnancy loss are Health Sciences, Durham, NC, USA PARTICIPANTS high around the time that pregnancies are clinically 5Department of Clinical Science, All Norwegian women that were pregnant between recognised. As a result, the total rate of recognised University of Bergen, Bergen, 2009-13. loss is sensitive to how early women recognise their Norway pregnancies. There are also differences across countries 6 MAIN OUTCOME MEASURE Department of Obstetrics and studies in distinguishing between miscarriage and and Gynecology, Haukeland Risk of miscarriage according to the woman’s age and University Hospital, Bergen, pregnancy history estimated by logistic regression. -

Gestational Diabetes Insipidus (GDI) Associated with Pre-Eclampsia
MOJ Women’s Health Case Report Open Access Gestational diabetes insipidus (GDI) associated with pre-eclampsia Abstract Volume 5 Issue 6 - 2017 Gestational diabetes insipidus (GDI) is a rare complication of pregnancy, usually developing in the third trimester and remitting spontaneously 4-6weeks post-partum. Afsoon Razavi, Muhammad Umair, Zehra It is mainly caused by excessive vasopressinase activity, an enzyme expressed by Tekin, Issac Sachmechi placental trophoblasts which metabolizes arginine vasopressin (AVP). The treatment Department of medicine, Icahn School of Medicine at Mount requires desmopressin. A 38year old G3P0A2 women with no significant medical Sinai/NYC Health+ Hospital/Queens, USA history was admitted to obstetrical service on 36th week of gestation due to significant malaise, anorexia, nausea, vomiting, polyuria, nocturia, and polydipsia, worsening Correspondence: Afsoon Razavi, MD, Department of in the 2weeks prior to presentation. Physical examination demonstrated decreased Medicine, Icahn School of Medicine at Mount Sinai/NYC skin turgor, hyperactive tendon reflexes and no pedal edema, and her blood pressure Health+Hospital/Queens, Diabetes center, 4th floor, Suit P-432, Pavilion building, Queens hospital center, 82-68 164th street, was 170/100mmHg, heart rate 67beats/min and weight 60kg (BMI 23.1kg/m2). Her Jamaica, New York, 11432, USA, Tel +8183843165, laboratory results a month prior to admission showed normal basic metabolic panel Email [email protected] and liver function tests. On admission she found to have urine osmolality112mOsmol/ kg (350-1000); serum osmolality 308mOsmol/kg (278-295); Urinalysis revealed Received: July 25, 2017 | Published: August 16, 2017 specific gravity less than 1005 with proteinuria. serum sodium 151mmol/L (135-145); potassium 4.1mmol/L (3.5-5.0); Cl 128mmol/L, urea 2.2 mmol/L (2.5-6.7), creatinine 1.4mg/dL, Bilirubin 1.3mg/dL, AST 1270 U/L, alkaline phosphatase, 717U/L, uric acid 10.1mg/dL and INR 1.1. -

Standards of Medical Care in Diabetes—2018
Diabetes Care Volume 41, Supplement 1, January 2018 S137 13. Management of Diabetes American Diabetes Association in Pregnancy: Standards of Medical Care in Diabetesd2018 Diabetes Care 2018;41(Suppl. 1):S137–S143 | https://doi.org/10.2337/dc18-S013 13. MANAGEMENT OF DIABETES IN PREGNANCY The American Diabetes Association (ADA) “Standards of Medical Care in Diabetes” includes ADA’s current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee, are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA’s clinical practice recommendations, please refer to the Standards of Care Introduction. Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC. DIABETES IN PREGNANCY The prevalence of diabetes in pregnancy has been increasing in the U.S. The majority is gestational diabetes mellitus (GDM) with the remainder primarily preexisting type 1 diabetes and type 2 diabetes. The rise in GDM and type 2 diabetes in parallel with obesity both in the U.S. and worldwide is of particular concern. Both type 1 diabetes and type 2 diabetes in pregnancy confer significantly greater maternal and fetal risk than GDM, with some differences according to type of diabetes as outlined below. In general, specific risks of uncontrolled diabetes in pregnancy include spontaneous abortion, fetal anomalies, preeclampsia, fetal demise, macrosomia, neonatal hy- poglycemia, and neonatal hyperbilirubinemia, among others. -

Screening for Gestational Diabetes Mellitus: a Systematic Review to Update the 2014 U.S
Evidence Synthesis Number 204 Screening for Gestational Diabetes Mellitus: A Systematic Review to Update the 2014 U.S. Preventive Services Task Force Recommendation Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 5600 Fishers Lane Rockville, MD 20857 www.ahrq.gov Contract No. HHSA-290-2015-00009-I Prepared by: Pacific Northwest Evidence-Based Practice Center Oregon Health & Science University Mail Code: BICC 3181 SW Sam Jackson Park Road Portland, OR 97239 www.ohsu.edu/epc University of Alberta Evidence-Based Practice Center 4-474 Edmonton Clinic Health Academy 11405 – 87 Avenue Edmonton, Alberta, Canada T6G 1C9 Investigators: Jennifer Pillay, MSc Lois Donovan, MD Samantha Guitard, MSc Bernadette Zakher, MD Christina Korownyk, MD Michelle Gates, PhD Allison Gates, PhD Ben Vandermeer, MSc Christina Bougatsos, MPH Roger Chou, MD Lisa Hartling, PhD AHRQ Publication No. 21-05273-EF-1 February 2021 This report is based on research conducted by the Pacific Northwest Evidence-based Practice Center (EPC) and the University of Alberta EPC under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. HHSA-290-2015-00009-I). The findings and conclusions in this document are those of the authors, who are responsible for its contents, and do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help health care decisionmakers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of health care services. -

Pregnancy-Related Conditions As Disabilities Under the ADA
Pregnancy-Related Conditions as Disabilities under the ADA Following passage of the Americans with Disabilities Act Amendments Act of 2008 (“ADAAA”),1 the legal landscape of pregnancy accommodation has changed dramatically.2 That the ADAAA has expanded coverage for non-pregnant individuals is beyond dispute: the statutory language of the ADAAA has broadened the term “disability” and makes it unequivocally clear that it should “be construed in favor of broad coverage of individuals.”3 Under the broadened definition, most pregnancy-related conditions are likely to be considered disabilities the employers will have to reasonably accommodate. The Pregnancy Discrimination Act (PDA) mandates that pregnant workers be treated the same as other workers with a similar ability or inability to work.4 This mandate means that pregnant women, who often experience diseases identical to those experienced in the general population, are to be afforded the same accommodations. For example, pregnant women frequently get carpal tunnel syndrome, and should receive the same breaks, job modifications, or supportive devices as non-pregnant employees with the syndrome. Otherwise, a nonsensical result occurs: a worker with carpal tunnel syndrome may qualify for ADA accommodation if the syndrome stems from any condition in the world other than pregnancy, but not if it stems from pregnancy. In addition, pregnant women often experience symptoms similar or identical to those experienced by non-pregnant workers. For example, if a non-pregnant worker with back problems that prohibits him from lifting more than 20 pounds for several months would have a qualifying disability under the ADA, then the same must be true for a pregnant woman suffering from back pain that requires her to request a lifting restriction. -

Am I at Risk for Gestational Diabetes?
Am I at risk for gestational diabetes? U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH Eunice Kennedy Shriver National Institute of Child Health and Human Development What is gestational diabetes? Gestational diabetes (pronounced jess-TAY-shun-ul die-uh-BEET-eez) is a type of high blood sugar that only pregnant women get. In fact, the word “gestational” means pregnant. If a woman gets high blood sugar when she’s pregnant, but she never had high blood sugar before, she has gestational diabetes. Between 2 percent and 10 percent of U.S. pregnancies are affected by the condition every year,1 making it one of the top health concerns related to pregnancy. If not treated, gestational diabetes can cause problems for mothers and babies, some of them serious. But there is good news. Most of the time, gestational diabetes goes away after the baby is born. The changes in your body that cause gestational diabetes normally occur only when you are pregnant. After the baby is born, your body goes back to normal and the condition goes away. Gestational diabetes is treatable, and the best outcomes result from careful management and control of blood sugar levels. The best way to control gestational diabetes is to find out you have it early and start treatment quickly. Treating gestational diabetes—even if you don’t have any symptoms or your symptoms are mild— greatly reduces health problems for mother and baby. Why do some women get gestational diabetes? Usually, the body breaks down much of the food you eat into a type of sugar, called glucose (pronounced GLOO-kos). -

Study of the Placental Attachment of Funiculus
International Journal of Anatomy and Research, Int J Anat Res 2017, Vol 5(1):3535-40. ISSN 2321-4287 Original Research Article DOI: https://dx.doi.org/10.16965/ijar.2017.107 STUDY OF THE PLACENTAL ATTACHMENT OF FUNICULUS UMBILICALIS IN NORMAL AND PRE-ECLAMPTIC PREGNANCIES AND ITS EFFECTS ON BIRTH WEIGHT Ankit Jain *1, Sonia Baweja 2, Rashmi Jain 3. *1 M.S., Ex. Resident, Department of Anatomy, Gandhi Medical College, Bhopal (M.P.), India. 2 M.S., Associate Professor, Department of Anatomy, Gandhi Medical College, Bhopal (M.P.), India. 3 M.D., Lab Head, Consultant Pathologist, SRL Diagnostics Ltd, Malviya Nagar, Bhopal (M.P.), India. ABSTRACT Introduction: Abnormalities in the insertion of umbilical cord is associated with a number of complications in pregnancy and these complications may adversely affect the fetus. The aim of this study was to evaluate the variations in the attachment of umbilical cord in normal and pre-eclamptic pregnancies and to assess the effects of variable cord insertions on fetal birth weight. Materials and Methods: Seventy placentae each of normotensive and pre-eclamptic pregnancies were studied (n=140). After delivery, weight of the baby was recorded by using weighing machine and the attachment of umbilical cord on placenta was observed. Results: In the present study, commonest site of insertion of umbilical cord was central (60%) in normal pregnancies, whereas in pre-eclamptic pregnancies, a common site of insertions of umbilical cord were central (37.14%) and/or eccentric (34.28%). Marginal cord insertions were found 2.11 times more in pre-eclamptic pregnancies as compared to normal pregnancies. -

Gestational Diabetes Key Points
DIABETES IN PATIENT SUBGROUP Gestational diabetes Key points Yael R Lefkovits C Treatment of diabetes mellitus in pregnancy reduces the risk of Zoe A Stewart perinatal complications, including pre-eclampsia, pregnancy- induced hypertension, caesarean delivery, large for gestational Helen R Murphy age babies and neonatal adiposity in women with gestational diabetes mellitus (GDM) Abstract C There is inadequate evidence to recommend universal One in six live births occur in women with diabetes mellitus, of which screening for GDM across all countries. Screening should be the most common type, accounting for approximately 87.5% of all dia- tailored to the clinical and economic needs of the region betes in pregnancy, is gestational diabetes mellitus (GDM). Maternal hyperglycaemia is one of the principle determinants of maternal C Continuous glucose monitoring provides a direct measure of e fetal complications in pregnancy in GDM. In particular, hyperglycae- fetal exposure to maternal blood glucose concentrations and mia is most commonly associated with increased rates of instrumental will be used increasingly in research and in clinical practice and/or operative delivery, pre-eclampsia, increased adiposity, macro- somia and infant birthweight >90th percentile. Large for gestational C The potential benefits of oral hypoglycaemic agents (metfor- age infants have an increased risk of birth complications, including min, glibenclamide) should be weighed against their uncertain shoulder dystocia and stillbirth. Maternal hyperglycaemia is also one long-term risks on the developing fetus of the factors most amenable to treatment during pregnancy. For most women with GDM, dietary and lifestyle modifications are suffi- cient to achieve glycaemic targets and optimal pregnancy outcomes. -

After Delivery
After Delivery Note to the Health Care Provider: Topics in this handout are discussed in Chapter 10 of the American Dietetic Association Guide to Gestational Diabetes Mellitus (1). When counseling women with gestational diabetes mellitus about postpartum issues, consider the following strategies: ■ Encourage the woman to have a 75-gram oral glucose tolerance test (OGTT) performed at 6 to 12 weeks’ postpartum to determine whether the diabetes remained after delivery or whether she is at risk of developing diabetes in the future. ■ Assist the woman in establishing realistic goals for postpartum weight management. Discuss the need to avoid fad diets and eating plans that promote rapid weight loss of more than 2 to 4 pounds (1 to 2 kg) per month. Breastfeeding women should avoid rapid weight loss, which may interfere with milk production (see the Breastfeeding handout). ■ Encourage the woman to eat a heart-healthy food plan by incorporating more nutrient-dense foods such as fruits, vegetables, and whole grains. Adequate calcium intake should also be stressed. Monounsaturated fats should be used in place of other fats, and saturated fats should be limited. Lean meats, vegetarian dishes, and fish high in omega-3 fatty acids should be selected instead of high-fat deep-fried items. ■ Encourage regular physical activity, and advise the woman on how to incorporate physical activity into her lifestyle. ■ Explain the importance of preconception counseling if the woman desires to have additional children. The health care provider should screen for diabetes or impaired glucose tolerance prior to conception. Explain that the first 12 weeks of gestation is the period of organogenesis and that optimal glycemic control during this time is critical. -

Gestational Diabetes, Me & My Baby Before the Baby
Gestational Diabetes, Me & My Baby Before the Baby Being diagnosed with gestational diabetes can be scary. Luckily, there are many things you can do to keep you and your baby healthy both during and after pregnancy. Go to all your prenatal levels. Ask your health care appointments so your provider for suggestions. health care provider can keep you and your baby Ask your health care healthy. provider how much weight gain is right for Gestational diabetes can cause you during pregnancy. your baby to be larger than normal when you are pregnant. Staying within your weight gain It may cause your baby to have range can help control your low blood sugar, yellowish skin blood sugar levels and keep your (jaundice), or trouble breathing baby healthy. at birth. Keeping your prenatal appointments and managing Check your blood sugar your blood sugar can prevent levels regularly and write these conditions. them down to bring to your appointments. Learn about changes you Make a commitment must make to your daily You may not have to use to breastfeeding before meals. medications if you check your blood sugar levels often and your baby is born. When you have gestational control them. Breastfeeding can help delay or diabetes, the sugar in your blood prevent diabetes in the future can become too high. Changing Take insulin or other for you and your baby. It can what you eat can help keep your medications as prescribed also help prevent obesity in your blood sugar at a safe level. It is to control your blood child. important to receive nutritional sugar.