A Review Article- Gestational Diabetes Mellitus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gestational Diabetes Insipidus, HELLP Syndrome and Eclampsia in a Twin Pregnancy: a Case Report
Journal of Perinatology (2010) 30, 144–145 r 2010 Nature Publishing Group All rights reserved. 0743-8346/10 $32 www.nature.com/jp PERINATAL/NEONATAL CASE PRESENTATION Gestational diabetes insipidus, HELLP syndrome and eclampsia in a twin pregnancy: a case report JL Woelk, RA Dombroski and PR Brezina Department of Obstetrics and Gynecology, East Carolina University, Greenville, NC, USA alanine aminotransferase, 65 U lÀ1; and lactate dehydrogenase, We report a case of eclampsia in a twin pregnancy complicated by HELLP 390 U lÀ1. A 24-h urine collection was begun to quantify proteinuria. syndrome and diabetes insipidus. This confluence of disease processes During the first night of hospitalization, the patient developed suggests that a modification of common magnesium sulfate treatment marked polyuria and polydipsia. Urine output increased to 1500 cc hÀ1 protocols may be appropriate in a certain subset of patients. by the early morning totaling 12 l for the entire night. Repeat Journal of Perinatology (2010) 30, 144–145; doi:10.1038/jp.2009.115 electrolytes were unchanged. The endocrinology service was then Keywords: diabetes insipidus ; eclampsia ; HELLP syndrome ; twin consulted for the management of presumptive DI. Therapy with the pregnancy ; magnesium sulfate administration of dDAVP (1-deamino-8-D-arginine vasopressin) orally twice daily was initiated. Serum osmolality was increased at 296 mOsm kgÀ1, and urine osmolality was decreased to 71 mOsm kgÀ1 Introduction with a specific gravity of 1.000. The 24-h urine results showed a total The association between diabetes insipidus (DI) and liver protein of 780 mg. The patient denied the previously reported dysfunction in a preeclamptic patient has been established in headaches, blurry vision and right upper quadrant pain, and her blood multiple case reports.1 This case is an important example that pressures remained 140 to 155 mmHg (systolic) and 80 to 95 mmHg illustrates how the pathophysiology of DI and the pharmacokinetics (diastolic), consistent with a diagnosis of mild preeclampsia. -

PLANNED OUT-OF-HOSPITAL BIRTH Approved 11/12/15
HEALTH EVIDENCE REVIEW COMMISSION (HERC) COVERAGE GUIDANCE: PLANNED OUT-OF-HOSPITAL BIRTH Approved 11/12/15 HERC COVERAGE GUIDANCE Planned out-of-hospital (OOH) birth is recommended for coverage for women who do not have high- risk coverage exclusion criteria as outlined below (weak recommendation). This coverage recommendation is based on the performance of appropriate risk assessments1 and the OOH birth attendant’s compliance with the consultation and transfer criteria as outlined below. Planned OOH birth is not recommended for coverage for women who have high risk coverage exclusion criteria as outlined below, or when appropriate risk assessments are not performed, or where the attendant does not comply with the consultation and transfer criteria as outlined below (strong recommendation). High-risk coverage exclusion criteria: Complications in a previous pregnancy: Maternal surgical history Cesarean section or other hysterotomy Uterine rupture Retained placenta requiring surgical removal Fourth-degree laceration without satisfactory functional recovery Maternal medical history Pre-eclampsia requiring preterm birth Eclampsia HELLP syndrome Fetal Unexplained stillbirth/neonatal death or previous death related to intrapartum difficulty Baby with neonatal encephalopathy Placental abruption with adverse outcome Complications of current pregnancy: Maternal Induction of labor Prelabor rupture of membranes > 24 hours 1 Pre-existing chronic hypertension; Pregnancy-induced hypertension with diastolic blood pressure greater than -
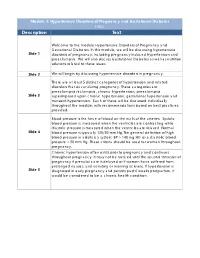
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text
Module 2: Hypertensive Disorders of Pregnancy and Gestational Diabetes FINAL Description Text Welcome to the module Hypertensive Disorders of Pregnancy and Gestational Diabetes. In this module, we will be discussing hypertensive Slide 1 disorders of pregnancy, including pregnancy induced hypertension and preeclampsia. We will also discuss Gestational Diabetes as well as nutrition solutions related to these issues Slide 2 We will begin by discussing hypertensive disorders in pregnancy. There are at least 5 distinct categories of hypertension and related disorders that occur during pregnancy. These categories are: preeclampsia/eclampsia, chronic hypertension, preeclampsia Slide 3 superimposed upon chronic hypertension, gestational hypertension and transient hypertension. Each of these will be discussed individually throughout the module, with recommendations based on best practices provided. Blood pressure is the force of blood on the walls of the arteries. Systolic blood pressure is measured when the ventricles are contracting while diastolic pressure is measured when the ventricles are relaxed. Normal Slide 4 blood pressure is typically 120/80 mm Hg.The general definition of high blood pressure in adults is a systolic BP > 140 mg HG or a diastolic blood pressure > 90 mm Hg. These criteria should be used for women throughout pregnancy. Chronic hypertension often exists prior to pregnancy and continues throughout pregnancy. It may not be noticed until the second trimester of pregnancy if prenatal care is delayed or if women have suffered from prolonged nausea and vomiting or morning sickness. If hypertension is Slide 5 diagnosed in early pregnancy and persists past 6 weeks postpartum, it would be considered to be a chronic health condition. -

Role of Maternal Age and Pregnancy History in Risk of Miscarriage
RESEARCH Role of maternal age and pregnancy history in risk of BMJ: first published as 10.1136/bmj.l869 on 20 March 2019. Downloaded from miscarriage: prospective register based study Maria C Magnus,1,2,3 Allen J Wilcox,1,4 Nils-Halvdan Morken,1,5,6 Clarice R Weinberg,7 Siri E Håberg1 1Centre for Fertility and Health, ABSTRACT Miscarriage and other pregnancy complications might Norwegian Institute of Public OBJECTIVES share underlying causes, which could be biological Health, PO Box 222 Skøyen, To estimate the burden of miscarriage in the conditions or unmeasured common risk factors. N-0213 Oslo, Norway Norwegian population and to evaluate the 2MRC Integrative Epidemiology associations with maternal age and pregnancy history. Unit at the University of Bristol, Introduction Bristol, UK DESIGN 3 Miscarriage is a common outcome of pregnancy, Department of Population Prospective register based study. Health Sciences, Bristol Medical with most studies reporting 12% to 15% loss among School, Bristol, UK SETTING recognised pregnancies by 20 weeks of gestation.1-4 4Epidemiology Branch, National Medical Birth Register of Norway, the Norwegian Quantifying the full burden of miscarriage is Institute of Environmental Patient Register, and the induced abortion register. challenging because rates of pregnancy loss are Health Sciences, Durham, NC, USA PARTICIPANTS high around the time that pregnancies are clinically 5Department of Clinical Science, All Norwegian women that were pregnant between recognised. As a result, the total rate of recognised University of Bergen, Bergen, 2009-13. loss is sensitive to how early women recognise their Norway pregnancies. There are also differences across countries 6 MAIN OUTCOME MEASURE Department of Obstetrics and studies in distinguishing between miscarriage and and Gynecology, Haukeland Risk of miscarriage according to the woman’s age and University Hospital, Bergen, pregnancy history estimated by logistic regression. -

Pre-Pregnancy Risk Factors for Severe Hyperemesis Gravidarum: Korean Population Based Cohort Study
life Article Pre-Pregnancy Risk Factors for Severe Hyperemesis Gravidarum: Korean Population Based Cohort Study Ho Yeon Kim 1, Geum Joon Cho 1,*, So Yeon Kim 1, Kyu-Min Lee 2, Ki Hoon Ahn 1 , Sung Won Han 2, Soon-Cheol Hong 1, Hyun Mee Ryu 3, Min-Jeong Oh 1, Hai-Joong Kim 1 and Seung Chul Kim 4,* 1 Department of Obstetrics and Gynecology, Korea University College of Medicine, 27 Inchonro, Seongbuk-gu, Seoul 02841, Korea; [email protected] (H.Y.K.); [email protected] (S.Y.K.); [email protected] (K.H.A.); [email protected] (S.-C.H.); [email protected] (M.-J.O.); [email protected] (H.-J.K.) 2 School of Industrial Management Engineering, Korea University, 145 Anam-ro, Anam-dong, Seongbuk-gu, Seoul 02841, Korea; [email protected] (K.-M.L.); [email protected] (S.W.H.) 3 Department of Obstetrics and Gynecology, CHA Bungdang Medical Center, CHA University School of Medicine, 59 Yatap-ro, Bundang-gu, Seongnam-si, Gyeonggi-do 13496, Korea; [email protected] 4 Department of Obstetrics and Gynecology, Pusan National University School of Medicine, 2 Busandaehak-ro 63beon-gil, Jangjeon 2(i)-dong, Geumjeong-gu, Busan 46241, Korea * Correspondence: [email protected] (G.J.C.); [email protected] (S.C.K.) Abstract: Hyperemesis gravidarum is known to be associated with poor perinatal outcomes. This study aimed to identify pre-pregnancy risk factors for hospital admission in women with hyperemesis gravidarum. We enrolled women who had delivered between 1 January 2013 and 31 December 2015, and had undergone a national health screening examination through the National Health Insurance Corporation 1–2 years before their first delivery. -

What You Need to Know About Morning Sickness What Is Morning Sickness? Morning Sickness Is Nausea And/Or Vomiting That Many Pregnant Women Experience
What You Need to Know About Morning Sickness What is morning sickness? Morning sickness is nausea and/or vomiting that many pregnant women experience. The term “morning sickness” is common, but it’s not correct, because many women have nausea and vomiting all day. The most important thing to know about nausea you may experience during your pregnancy is it’s normal. According to the American Pregnancy Association, more than 50% of pregnant women have nausea and/or vomiting. Although it’s most common during the first trimester, it’s possible to feel sick throughout the entire nine months of your pregnancy. For some women, feeling nauseous and/or throwing up are among the first symptoms of pregnancy. Most women start having nausea and/or vomiting around the sixth week of their first trimester. And some women notice their symptoms disappear around the 12th week of pregnancy or their second trimester. In general, nausea when pregnant isn’t harmful to you or the baby. However, if you can’t keep water or food down for long periods, then it can be dangerous, and you should talk to your provider about it. Common symptoms • Nausea • Vomiting • Feeling sick • Not being able to handle specific odors or foods Extreme morning sickness: Hyperemesis gravidarum Estimates are that 3% of pregnant women have hyperemesis gravidarum. This extreme nausea, vomiting and weight loss during pregnancy can be harmful to you and the baby, so you should talk to your doctor right away. If you’re not able to keep food or water down, then you could become malnourished and dehydrated. -

Guide to Learning in Maternal-Fetal Medicine
GUIDE TO LEARNING IN MATERNAL-FETAL MEDICINE First in Women’s Health The Division of Maternal-Fetal Medicine of The American Board of Obstetrics and Gynecology, Inc. 2915 Vine Street Dallas, TX 75204 Direct questions to: ABOG Fellowship Department 214.871.1619 (Main Line) 214.721.7526 (Fellowship Line) 214.871.1943 (Fax) [email protected] www.abog.org Revised 4/2018 1 TABLE OF CONTENTS I. INTRODUCTION ........................................................................................................................ 3 II. DEFINITION OF A MATERNAL-FETAL MEDICINE SUBSPECIALIST .................................... 3 III. OBJECTIVES ............................................................................................................................ 3 IV. GENERAL CONSIDERATIONS ................................................................................................ 3 V. ENDOCRINOLOGY OF PREGNANCY ..................................................................................... 4 VI. PHYSIOLOGY ........................................................................................................................... 6 VII. BIOCHEMISTRY ........................................................................................................................ 9 VIII. PHARMACOLOGY .................................................................................................................... 9 IX. PATHOLOGY ......................................................................................................................... -

Correlation of Estradiol and Estriol Serum
2019 Skin Diseases & Skin Care Extended Abstract Vol.4 No.3 Correlation of Estradiol and Estriol Serum Levels to Melasma Severity in Pregnant Women Aunur Rofiq* , SHW Tantari, A Widiatmoko and Dyah Ayu Savitri Dermatology and Venereology, Faculty of Medicine, Brawijaya University, RSUD Dr Saiful Anwar Malang, Indonesia Email: [email protected] ABSTRACT Melasma is also known as chloasma or mask of potency of estriol; therefore, estradiol is said to be pregnancy because it shows during pregnancy as a the main estrogen . A study by Gopichandani et al. symmetrical hyperpigmented lesion Melasma supported the hypothesis that the main patients in Indonesia was estimated to be 0.2–4% pathogenesis of melasma was estradiol. НLs was of all dermatology patients . In Saiful Anwar proven by the high estradiol level in pregnant General Hospital Malang, East Java, Indonesia, women with melasma compared to those without melasma was found in 338 of 9736 dermatology melasma. Нe other forms of estrogen such as patients per year (3.4%) in 2014, and is the seventh estriol and estrone also Defected the cytoplasm and most common diagnosis in dermatology clinic. In the main estrogen receptor known to be expressed 2015, melasma incidence was declined to 226 of in the melanocyte . Especially in the third 8310 patients per year and not included in the top trimester, the high level of estriol and estradiol was ten most common dermatology diagnoses. Нe related to the high level of MSH, which caused current data from Saiful Anwar General Hospital tyrosinase and dopachrome tautomerase dermatology clinic in 2016 stated that melasma production; this sequence led to melanogenesis and patient was 185 of 7945 patients per year (2.3%). -

Gestational Diabetes Insipidus (GDI) Associated with Pre-Eclampsia
MOJ Women’s Health Case Report Open Access Gestational diabetes insipidus (GDI) associated with pre-eclampsia Abstract Volume 5 Issue 6 - 2017 Gestational diabetes insipidus (GDI) is a rare complication of pregnancy, usually developing in the third trimester and remitting spontaneously 4-6weeks post-partum. Afsoon Razavi, Muhammad Umair, Zehra It is mainly caused by excessive vasopressinase activity, an enzyme expressed by Tekin, Issac Sachmechi placental trophoblasts which metabolizes arginine vasopressin (AVP). The treatment Department of medicine, Icahn School of Medicine at Mount requires desmopressin. A 38year old G3P0A2 women with no significant medical Sinai/NYC Health+ Hospital/Queens, USA history was admitted to obstetrical service on 36th week of gestation due to significant malaise, anorexia, nausea, vomiting, polyuria, nocturia, and polydipsia, worsening Correspondence: Afsoon Razavi, MD, Department of in the 2weeks prior to presentation. Physical examination demonstrated decreased Medicine, Icahn School of Medicine at Mount Sinai/NYC skin turgor, hyperactive tendon reflexes and no pedal edema, and her blood pressure Health+Hospital/Queens, Diabetes center, 4th floor, Suit P-432, Pavilion building, Queens hospital center, 82-68 164th street, was 170/100mmHg, heart rate 67beats/min and weight 60kg (BMI 23.1kg/m2). Her Jamaica, New York, 11432, USA, Tel +8183843165, laboratory results a month prior to admission showed normal basic metabolic panel Email [email protected] and liver function tests. On admission she found to have urine osmolality112mOsmol/ kg (350-1000); serum osmolality 308mOsmol/kg (278-295); Urinalysis revealed Received: July 25, 2017 | Published: August 16, 2017 specific gravity less than 1005 with proteinuria. serum sodium 151mmol/L (135-145); potassium 4.1mmol/L (3.5-5.0); Cl 128mmol/L, urea 2.2 mmol/L (2.5-6.7), creatinine 1.4mg/dL, Bilirubin 1.3mg/dL, AST 1270 U/L, alkaline phosphatase, 717U/L, uric acid 10.1mg/dL and INR 1.1. -

Standards of Medical Care in Diabetes—2018
Diabetes Care Volume 41, Supplement 1, January 2018 S137 13. Management of Diabetes American Diabetes Association in Pregnancy: Standards of Medical Care in Diabetesd2018 Diabetes Care 2018;41(Suppl. 1):S137–S143 | https://doi.org/10.2337/dc18-S013 13. MANAGEMENT OF DIABETES IN PREGNANCY The American Diabetes Association (ADA) “Standards of Medical Care in Diabetes” includes ADA’s current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee, are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA’s clinical practice recommendations, please refer to the Standards of Care Introduction. Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC. DIABETES IN PREGNANCY The prevalence of diabetes in pregnancy has been increasing in the U.S. The majority is gestational diabetes mellitus (GDM) with the remainder primarily preexisting type 1 diabetes and type 2 diabetes. The rise in GDM and type 2 diabetes in parallel with obesity both in the U.S. and worldwide is of particular concern. Both type 1 diabetes and type 2 diabetes in pregnancy confer significantly greater maternal and fetal risk than GDM, with some differences according to type of diabetes as outlined below. In general, specific risks of uncontrolled diabetes in pregnancy include spontaneous abortion, fetal anomalies, preeclampsia, fetal demise, macrosomia, neonatal hy- poglycemia, and neonatal hyperbilirubinemia, among others. -

Screening for Gestational Diabetes Mellitus: a Systematic Review to Update the 2014 U.S
Evidence Synthesis Number 204 Screening for Gestational Diabetes Mellitus: A Systematic Review to Update the 2014 U.S. Preventive Services Task Force Recommendation Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 5600 Fishers Lane Rockville, MD 20857 www.ahrq.gov Contract No. HHSA-290-2015-00009-I Prepared by: Pacific Northwest Evidence-Based Practice Center Oregon Health & Science University Mail Code: BICC 3181 SW Sam Jackson Park Road Portland, OR 97239 www.ohsu.edu/epc University of Alberta Evidence-Based Practice Center 4-474 Edmonton Clinic Health Academy 11405 – 87 Avenue Edmonton, Alberta, Canada T6G 1C9 Investigators: Jennifer Pillay, MSc Lois Donovan, MD Samantha Guitard, MSc Bernadette Zakher, MD Christina Korownyk, MD Michelle Gates, PhD Allison Gates, PhD Ben Vandermeer, MSc Christina Bougatsos, MPH Roger Chou, MD Lisa Hartling, PhD AHRQ Publication No. 21-05273-EF-1 February 2021 This report is based on research conducted by the Pacific Northwest Evidence-based Practice Center (EPC) and the University of Alberta EPC under contract to the Agency for Healthcare Research and Quality (AHRQ), Rockville, MD (Contract No. HHSA-290-2015-00009-I). The findings and conclusions in this document are those of the authors, who are responsible for its contents, and do not necessarily represent the views of AHRQ. Therefore, no statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. The information in this report is intended to help health care decisionmakers—patients and clinicians, health system leaders, and policymakers, among others—make well-informed decisions and thereby improve the quality of health care services. -

The Effect of Hyperemesis Gravidarum on Pregnancy Outcomes Hiperemezis Gravidarumun Gebelik Sonuçlarına Etkisi
JOURNAL OF Journal of Contemporary CONTEMPORARY MEDICINE Medicine DOI: 10.16899/jcm.870631 J Contemp Med 2021;11(4):428-432 Orjinal Araştırma / Original Article The Effect of Hyperemesis Gravidarum on Pregnancy Outcomes Hiperemezis Gravidarumun Gebelik Sonuçlarına Etkisi Zekiye Soykan Sert1 1Department of Gynecology and Obstetrics, Aksaray University Education and Research Hospital, Aksaray, Turkey Abstract Öz Objective: We evaluated the clinical characteristics of the patients Amaç: Hiperemezis gravidarum (HG) tanısı ile takip edilen hastaların followed with the diagnosis of hyperemesis gravidarum (HG). We klinik özelliklerini değerlendirdik. Bu hastalarda HG'nin gebelik aimed to determine the effects of HG on pregnancy outcomes in sonuçları üzerindeki etkilerini belirlemeyi amaçladık. this study. Material and Method: This retrospective study was conducted in Gereç ve Yöntem: Bu retrospektif çalışma 2018-2020 yılları arasında the department of obstetrics and gynecology between January hastanemiz kadın hastalıkları bölümünde gerçekleştirildi. Çalışma 2018–2020. The study group consisted of pregnant women who grubu, 20. gebelik haftasından önce HG tanısı alan ve hastanemizde were diagnosed with HG before the 20th gestational week and tedavi edilerek doğum yapılan gebelerden oluşturuldu. Hastalar HG were treated and delivered at our hospital. The patients were varlığına göre iki gruba ayrıldı. Her iki grup plasental disfonksiyon divided into two groups based on the presence of HG. Both groups were compared in terms of placental dysfunction and ve yenidoğan sonuçları açısından karşılaştırıldı. HG'nin şiddeti newborn outcomes. The severity of the HG was assessed and değerlendirildi ve sınıflandırıldı. Hafif ve ağır vakaları karşılaştıran HG classified. A sub-analysis of the HG group comparing mild and grubunun bir alt analizi yapıldı.