Experiment 3 Gamma-Ray Spectroscopy Using Nai(Tl)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ZEPLIN I—The UKDM Single Phase Xenon Experiment at Boulby Neil Spooner∗ Department of Physics and Astronomy University of Sheffield, Hounsfield Road, Sheffield, S3 7RH, UK
ZEPLIN I—The UKDM Single Phase Xenon Experiment at Boulby Neil Spooner∗ Department of Physics and Astronomy University of Sheffield, Hounsfield Road, Sheffield, S3 7RH, UK I briefly review the ZEPLIN I liquid xenon detector of the UKDM collaboration. 1. ZEPLIN I Detector The ZEPLIN I detector is a single phase, 3.6 kg fiducial mass, liquid xenon scintillation detector built by the UKDM Collaboration (RAL-Sheffield-ICSTM) and running at the Boulby underground site (see Figure 1). The target mass is housed in a Cu-101 copper vessel, shaped to maximise light collection, which provides a uniform temperature environment through the use of a cryo- liquid jacket. The fiducial volume of xenon is surrounded bya5mmPTFE reflector, giving diffuse scattering of the 175 nm scintillation photons to maximise light collection. This volume is viewed through 3 mm silica windows by three quartz windowed photomultipliers through optically isolated Œturrets¹ of liquid xenon. These act both as light guides and passive shielding for the X-ray emission from the photomultipliers. The novel use of the xenon turrets arose from experience with NaI detectors where solid high-grade silica is used for light guides and shields. However, the extensive use of such material for liquid xenon is precluded due to the absorption of the 175 nm photons. Scintillation light produced in the turret regions is seen predominantly by the nearest photomultiplier which allows the rejection of the photomultiplier X-ray events and the definition of a fiducial volume through a comparison of the light seen by each tube. Purification of the xenon is performed using Oxysorb ion exchange columns with additional purification by vacuum pumping on frozen xenon and subsequent fractionation of the xenon gas. -

Experimental Γ Ray Spectroscopy and Investigations of Environmental Radioactivity
Experimental γ Ray Spectroscopy and Investigations of Environmental Radioactivity BY RANDOLPH S. PETERSON 216 α Po 84 10.64h. 212 Pb 1- 415 82 0- 239 β- 01- 0 60.6m 212 1+ 1630 Bi 2+ 1513 83 α β- 2+ 787 304ns 0+ 0 212 α Po 84 Experimental γ Ray Spectroscopy and Investigations of Environmental Radioactivity Randolph S. Peterson Physics Department The University of the South Sewanee, Tennessee Published by Spectrum Techniques All Rights Reserved Copyright 1996 TABLE OF CONTENTS Page Introduction ....................................................................................................................4 Basic Gamma Spectroscopy 1. Energy Calibration ................................................................................................... 7 2. Gamma Spectra from Common Commercial Sources ........................................ 10 3. Detector Energy Resolution .................................................................................. 12 Interaction of Radiation with Matter 4. Compton Scattering............................................................................................... 14 5. Pair Production and Annihilation ........................................................................ 17 6. Absorption of Gammas by Materials ..................................................................... 19 7. X Rays ..................................................................................................................... 21 Radioactive Decay 8. Multichannel Scaling and Half-life ..................................................................... -

Muon Decay 1
Muon Decay 1 LIFETIME OF THE MUON Introduction Muons are unstable particles; otherwise, they are rather like electrons but with much higher masses, approximately 105 MeV. Radioactive nuclear decays do not release enough energy to produce them; however, they are readily available in the laboratory as the dominant component of the cosmic ray flux at the earth’s surface. There are two types of muons, with opposite charge, and they decay into electrons or positrons and two neutrinos according to the rules + + µ → e νe ν¯µ − − µ → e ν¯e νµ . The muon decay is a radioactiveprocess which follows the usual exponential law for the probability of survival for a given time t. Be sure that you understand the basis for this law. The goal of the experiment is to measure the muon lifetime which is roughly 2 µs. With care you can make the measurement with an accuracy of a few percent or better. In order to achieve this goal in a conceptually simple way, we look only at those muons that happen to come to rest inside our detector. That is, we first capture a muon and then measure the elapsed time until it decays. Muons are rather penetrating particles, they can easily go through meters of concrete. Nevertheless, a small fraction of the muons will be slowed down and stopped in the detector. As shown in Figure 1, the apparatus consists of two types of detectors. There is a tank filled with liquid scintillator (a big metal box) viewed by two photomultiplier tubes (Left and Right) and two plastic scintillation counters (flat panels wrapped in black tape), each viewed by a photomul- tiplier tube (Top and Bottom). -

1. Gamma-Ray Detectors for Nondestructive Analysis P
1. GAMMA-RAY DETECTORS FOR NONDESTRUCTIVE ANALYSIS P. A. Russo and D. T. Vo I. INTRODUCTION AND OVERVIEW Gamma rays are used for nondestructive quantitative analysis of nuclear material. Knowledge of both the energy of the gamma ray and its rate of emission from the unknown mass of nuclear material is required to interpret most measurements of nuclear material quantities. Therefore, detection of gamma rays for nondestructive analysis of nuclear materials requires both spectroscopy capability and knowledge of absolute specific detector response. Some techniques nondestructively quantify attributes other than nuclear material mass, but all rely on the ability to distinguish elements or isotopes and measure the relative or absolute yields of their corresponding radiation signatures. All require spectroscopy and most require high resolution. Therefore, detection of gamma rays for quantitative nondestructive analysis (NDA) of the mass or of other attributes of nuclear materials requires spectroscopy. A previous book on gamma-ray detectors for NDA1 provided generic descriptions of three detector categories: inorganic scintillation detectors, semiconductor detectors, and gas-filled detectors. This report described relevant detector properties, corresponding spectral characteristics, and guidelines for choosing detectors for NDA. The current report focuses on significant new advances in detector technology in these categories. Emphasis here is given to those detectors that have been developed at least to the stage of commercial prototypes. The type of NDA application – fixed installation in a count room, portable measurements, or fixed installation in a processing line or other active facility (storage, shipping/receiving, etc.) – influences the choice of an appropriate detector. Some prototype gamma-ray detection techniques applied to new NDA approaches may revolutionize how nuclear materials are quantified in the future. -

Scintillation Detectors for X-Rays
INSTITUTE OF PHYSICS PUBLISHING MEASUREMENT SCIENCE AND TECHNOLOGY Meas. Sci. Technol. 17 (2006) R37–R54 doi:10.1088/0957-0233/17/4/R01 REVIEW ARTICLE Scintillation detectors for x-rays Martin Nikl Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnicka 10, 162 53 Prague, Czech Republic Received 23 March 2005 Published 10 February 2006 Online at stacks.iop.org/MST/17/R37 Abstract Recent research in the field of phosphor and scintillator materials and related detectors is reviewed. After a historical introduction the fundamental issues are explained regarding the interaction of x-ray radiation with a solid state. Crucial parameters and characteristics important for the performance of these materials in applications, including the employed measurement methods, are described. Extended description of the materials currently in use or under intense study is given. Scintillation detector configurations are further briefly overviewed and selected applications are mentioned in more detail to provide an illustration. Keywords: luminescence intensity, luminescence kinetics, light detection, x-ray detection, scintillators, phosphors, traps and material imperfections (Some figures in this article are in colour only in the electronic version) 1. Introduction development of phosphor and scintillator materials to be used in their exploitation. It was 110 years ago in November 1895 that Wilhelm Conrad It is to be noticed that for registration of x-ray the so- Roentgen noticed the glow of a barium platino-cyanide screen, called direct registration principle is widely used, in which the placed next to his operating discharge tube, and discovered new incoming radiation is directly converted into electrical current invisible and penetrating radiation [1], which was named x-ray in a semiconducting material. -

Nuclear Engineering and Technology 49 (2017) 1489E1494
Nuclear Engineering and Technology 49 (2017) 1489e1494 Contents lists available at ScienceDirect Nuclear Engineering and Technology journal homepage: www.elsevier.com/locate/net Original Article Large-volume and room-temperature gamma spectrometer for environmental radiation monitoring * Romain Coulon , Jonathan Dumazert, Tola Tith, Emmanuel Rohee, Karim Boudergui CEA, LIST, F-91191 Gif-sur-Yvette Cedex, France article info abstract Article history: The use of a room-temperature gamma spectrometer is an issue in environmental radiation monitoring. Received 29 April 2016 To monitor radionuclides released around a nuclear power plant, suitable instruments giving fast and Received in revised form reliable information are required. High-pressure xenon (HPXe) chambers have range of resolution and 15 May 2017 efficiency equivalent to those of other medium resolution detectors such as those using NaI(Tl), CdZnTe, Accepted 4 June 2017 and LaBr :Ce. An HPXe chamber could be a cost-effective alternative, assuming temperature stability and Available online 3 July 2017 3 reliability. The CEA LIST actively studied and developed HPXe-based technology applied for environ- mental monitoring. Xenon purification and conditioning was performed. The design of a 4-L HPXe de- Keywords: Xenon tector was performed to minimize the detector capacitance and the required power supply. Simulations fi Spectrometry were done with the MCNPX2.7 particle transport code to estimate the intrinsic ef ciency of the HPXe Environmental detector. A behavioral study dealing with ballistic deficits and electronic noise will be utilized to provide Detector perspective for further analysis. Radiation © 2017 Korean Nuclear Society, Published by Elsevier Korea LLC. This is an open access article under the Ionization chamber CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). -

Neutrino Detection with Liquid Scintillator Detectors
Neutrino Detection with Liquid Scintillator Detectors Minfang Yeh Neutrino and Nuclear Chemistry Chemistry/Instrumentation BNL VIRTUAL SYMPOSIUM FOR ETI/MTV CONSORTIA, 2021 Neutrino and Nuclear Chemistry at BNL since 1960+ 37 37 - HOMESTAKE 615t Cl + νe → Ar + e 71 71 - Gallex 30-t Ga + νe → Ge + e 780t D2O CC/NC SNO 200-kt H2O or 37-kt LAr LBNE (DUNE) 120-t 8% In-LS LENS 200-t 0.1% Gd-LS Daya Bay 780t 0.3% Nd/Te-LS SNO+ 6Li, 10B or Gd doped LS PROSPECT/LZ Metal-doped WbLS and 0νββ, dark-matter, AIT- plastic scintillator resins NEO, medical Water-based LS Scintillator Radiochemical Cerenkov 1960 1970 1980 1990 2000 2010 2020 M Yeh, BNL VIRTUAL SYMPOSIUM 2 LZ (Gd-doped) Dark Matter BNL-ν-Map SD, USA by liquid scintillator detectors AIT-NEO (WbLS) Boulby, UK JSNS2 (Gd-doped) SNO+ (Te-doped) Kamioka, Japan ANNIE Sudbury Canada (WbLS) BNL FNAL Daya Bay (Gd- doped), China PROSPECT (6Li-doped ), ORNL, TN, USA Cover a wide range of physics topics! neutrinos, dark matter, 0νββ, nonproliferation, medical physics,… M Yeh, BNL VIRTUAL SYMPOSIUM 3 Scintillation Mechanism S. Hans, J. Cumming, R. Rosero, S. Gokhale, R. Diaz, C. Camilo, M. Yeh, Light-yield quenching and remediation in liquid scintillator detectors, 2020 JINST 15 P12020 fast slow Stokes shift, photon-yield, timing structure, and C/H density determine the detector responses M Yeh, BNL VIRTUAL SYMPOSIUM 4 Scintillator Components C. Buck and M. Yeh, J. Phys. G: Nucl. Part. Phys. 43 093001 (2016) 200tons of Daya Bay Gd-LS produced in 2010; stable since production. -

Cosmic Ray Detector Hardware
Cosmic Ray Detector Hardware How it detects cosmic rays, what it measures and how to use it… Matthew Jones Purdue University 2012 QuarkNet Summer Workshop 1 What are Cosmic Rays? • Mostly muons down here… • Why are they called “rays”? – Purely historical • How can we detect them? – Muons are like heavy electrons – They have an electric charge – “Ionizing radiation”… – Some of their energy is transferred to the electrons in the material the move through – That’s what we detect… 2 Detecting Ionizing Radiation Cloud Chamber Solid State Geiger Counters Detectors Bubble Chamber Radiation creates Ion Chambers electron/hole pairs in Ionization initiates a silicon or germanium physical change in a that allow a current to gas or liquid. Wire Chambers flow. Photographic Film Crystal Scintillator GEM Detectors Photographic Organic An electric field does Emulsion Scintillator WORK on ionized gas Recombination of atoms to produce a Ionization initiates a electrons and ions voltage pulse. chemical reaction. produces light! 3 Plastic Scintillator 4 Plastic Scintillator • See, for example, Saint-Gobain, Inc. • Clear plastic traps light by total internal reflection. • Doped with a secret chemical that emits light when ionized, but does not re-absorb it. • Easy to cut, polish, bend, glue… • How much light is produced? – A muon travelling through 1 cm of plastic scintillator might produce about a thousand photons – Most of them would be blue – They bounce around inside the scintillator until they either escape or are absorbed • Usually wrapped in tin foil or white paper and then in black plastic or opaque paper to keep other light out. 5 The Cosmic Ray Detector Plastic scintillator wrapped in white paper and black plastic. -
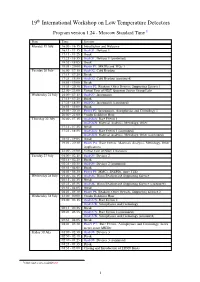
19 International Workshop on Low Temperature Detectors
19th International Workshop on Low Temperature Detectors Program version 1.24 - Moscow Standard Time 1 Date Time Session Monday 19 July 16:00 - 16:15 Introduction and Welcome 16:15 - 17:15 Oral O1: Devices 1 17:15 - 17:25 Break 17:25 - 18:55 Oral O1: Devices 1 (continued) 18:55 - 19:05 Break 19:05 - 20:00 Poster P1: MKIDs and TESs 1 Tuesday 20 July 16:00 - 17:15 Oral O2: Cold Readout 17:15 - 17:25 Break 17:25 - 18:55 Oral O2: Cold Readout (continued) 18:55 - 19:05 Break 19:05 - 20:30 Poster P2: Readout, Other Devices, Supporting Science 1 22:00 - 23:00 Virtual Tour of NIST Quantum Sensor Group Labs Wednesday 21 July 16:00 - 17:15 Oral O3: Instruments 17:15 - 17:25 Break 17:25 - 18:55 Oral O3: Instruments (continued) 18:55 - 19:05 Break 19:05 - 20:30 Poster P3: Instruments, Astrophysics and Cosmology 1 20:00 - 21:00 Vendor Exhibitor Hour Thursday 22 July 16:00 - 17:15 Oral O4A: Rare Events 1 Oral O4B: Material Analysis, Metrology, Other 17:15 - 17:25 Break 17:25 - 18:55 Oral O4A: Rare Events 1 (continued) Oral O4B: Material Analysis, Metrology, Other (continued) 18:55 - 19:05 Break 19:05 - 20:30 Poster P4: Rare Events, Materials Analysis, Metrology, Other Applications 22:00 - 23:00 Virtual Tour of NIST Cleanoom Tuesday 27 July 01:00 - 02:15 Oral O5: Devices 2 02:15 - 02:25 Break 02:25 - 03:55 Oral O5: Devices 2 (continued) 03:55 - 04:05 Break 04:05 - 05:30 Poster P5: MMCs, SNSPDs, more TESs Wednesday 28 July 01:00 - 02:15 Oral O6: Warm Readout and Supporting Science 02:15 - 02:25 Break 02:25 - 03:55 Oral O6: Warm Readout and Supporting -

Study of Electron Emissions of Some Mass Separated Fission Product Activities James Paul Adams Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1972 Study of electron emissions of some mass separated fission product activities James Paul Adams Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Nuclear Commons Recommended Citation Adams, James Paul, "Study of electron emissions of some mass separated fission product activities " (1972). Retrospective Theses and Dissertations. 5880. https://lib.dr.iastate.edu/rtd/5880 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This dissertation was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1. The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. -

Supplement to The
Hahn-Meitner-Institut Berlin Supplement to the Annual Report 2001 Berlin 2002 Supplement Index Publications 3 Structural Research 4 Solar Energy Research 21 Information Technology 30 Conference Contributions / Invited Lectures 31 Structural Research 32 Solar Energy Research 62 Information Technology 77 Technology Transfer / Patents 79 Academic Education 83 Courses 84 Exams 87 Co-operation Partners and Guests 89 Structural Research 90 Solar Energy Research 99 Information Technology 103 External Funding 105 Structural Research 106 Solar Energy Research 108 Participation in External Scientific Bodies and Committees 111 Miscellaneous 115 Awards / Exhibitions / Fairs / Organization of Conferences and Meetings / Events 1. Edition June 2002 Supplement of the Annual Report 2001 HMI-B 585 Hahn-Meitner-Institut Berlin GmbH Glienicker Str. 100 D-14109 Berlin (Wannsee) Coordination: Maren Achilles Phone: +49 – (0)30 – 8062 2668 Fax: +49 – (0)30 – 8062 2047 E-mail: [email protected] A 2 HMI Annual Report 2001 Publications 2001 Publications HMI Annual Report 2001 A 3 Publications 2001 Structural Research Department SF1 Pappas, C.; Kischnik, R.; Mezei, F. Wide angle NSE : the spectrometer SPAN at Instruments and Methods BENSC Physica B 297 (2001) 14-17 Pappas, C.; Mezei, F. Reviewed Publications How to achieve high intensity in NSE spectros- copy? BENSC-Activities Proceedings of the ILL Millenium Workshop, 2001, p.318 Ehlers, G.; Farago, B;. Pappas, C; Mezei, F. A new IN11 with an almost 35 times higher Peters, J.; Treimer, W. counting rate than that of IN11C Bloch walls in a nickel single crystal Proceedings of the ILL Millenium Workshop, 2001 p. Phys. Rev. B 64 (2001) 214415 – 214422 316 Scheffer, M.; Rouijaa, M.; Suck, J.-B.; Sterzel, R.; Fitzsimmons, M. -

Gamma Ray Spectroscopy
Gamma Ray Spectroscopy Ian Rittersdorf Nuclear Engineering & Radiological Sciences [email protected] March 20, 2007 Rittersdorf Gamma Ray Spectroscopy Contents 1 Abstract 3 2 Introduction & Objectives 3 3 Theory 4 3.1 Gamma-Ray Interactions . 5 3.1.1 Photoelectric Absorption . 5 3.1.2 Compton Scattering . 6 3.1.3 Pair Production . 8 3.2 Detector Response Function . 9 3.3 Complications in the Response Function . 11 3.3.1 Secondary Electron Escape . 11 3.3.2 Bremsstrahlung Escape . 12 3.3.3 Characteristic X-Ray Escape . 12 3.3.4 Secondary Radiations Created Near the Source . 13 3.3.5 Effects of Surrounding Materials . 13 3.3.6 Summation Peaks . 14 3.4 Semiconductor Diode Detectors . 15 3.5 High Purity Germanium Semiconductor Detectors . 17 3.5.1 HPGe Geometry . 18 3.6 Germanium Detector Setup . 18 3.7 Energy Resolution . 19 3.8 Background Radiation . 20 4 Equipment List 21 5 Setup & Settings 21 6 Analysis 23 6.1 Prominent Peak Information . 24 6.2 Calibration Curve . 24 6.3 Experiment Part 6 – 57Co ............................ 26 6.4 Experiment Part 6 – 60Co ............................ 28 6.5 Experiment Part 6 – 137Cs............................ 29 6.6 Experiment Part 6 – 22Na ............................ 31 6.7 Experiment Part 6 – 133Ba............................ 32 6.8 Experiment Part 6 – 109Cd............................ 34 6.9 Experiment Part 6 – 54Mn............................ 35 6.10 Energy Resolution . 37 6.11 Background Analysis . 39 1 Rittersdorf Gamma Ray Spectroscopy 7 Conclusions 43 Appendices i A 57Co Decay Scheme i B 60Co Decay Scheme ii C 137Cs Decay Scheme iii D 22Na Decay Scheme iv E 133Ba Decay Scheme v F 109Cd Decay Scheme vi G 54Mn Decay Scheme vii H HPGe Detector Apparatus viii I Raw Gamma-Ray Spectra ix References ix 2 Rittersdorf Gamma Ray Spectroscopy 1 Abstract In lab, a total of eight spectra were measured.