Sultopride Hydrochloride | Medchemexpress
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Letters to the Editor
Letters to the Editor Letters to the Editor Need for a New Framework to Understand lactin levels were high (115 mg/L; normal range, 12–30 mg/L). the© MechanismCopyright of All Antipsychotics 2000 PhysiciansAttempts Postgraduate to reduce dosage below Press, 80 mg/day resultedInc. in relapse, with good recovery when dosage was restored. Sir: The advent of atypical neuroleptics has transformed the A SPECT study was performed using a dual-headed camera pharmacologic treatment of schizophrenia. The advent of single and 185 MBq of 123-Iodobenzamide (IBZM). The specific-to- photon emission computed tomography (SPECT) and positron nonspecific binding ratio was measured at 90 minutes postinjec- emission tomography (PET) neuroreceptor imaging makes it tion. The SPECT was performed a week after a PET analysis possible to link biochemical events in the human brain to their was kindly performed in Dr. Kapur’s laboratory while the pa- clinical consequences. Remington and Kapur1 (supplement 10, tient received 90 mg/day of haloperidol. No detectable recep- 1999) have proposed an interesting model based on studies us- tors at this dosage were found with either technique. ing PET that takes in account the serotonin-2/dopamine-2 Our findings lead us to wonder whether there exists a sub- group of chronic patients resistant to conventional and some (5-HT2/D2) occupancy threshold: conventional antipsychotics atypical neuroleptics in normal doses, but who may respond have low 5-HT2/high D2 ratios, olanzapine and risperidone have well to conventional neuroleptics in high doses. Despite a high high 5-HT2/high D2 ratios, clozapine has a high 5-HT2/low D2 2,3 One personal copy maylevel be of printed striatal D occupancy, our patient did not present with ratio, and quetiapine has a low 5-HT2/low D2 ratio. -

Pharmacologyonline 2: 971-1020 (2009) Newsletter Gabriella Galizia
Pharmacologyonline 2: 971-1020 (2009) Newsletter Gabriella Galizia THE TREATMENT OF THE SCHIZOPHRENIA: AN OVERVIEW Gabriella Galizia School of Pharmacy,University of Salerno, Italy e-mail: [email protected] Summary The schizophrenia is a kind of psychiatric disease, characterized by a course longer than six months (usually chronic or relapsing), by the persistence of symptoms of alteration of mind, behaviour and emotion, with such a seriousness to limitate the normal activity of a person. The terms antipsychotic and neuroleptic define a group of medicine principally used to treat schizophrenia, but they are also efficacious for other psychosis and in states of psychic agitation. The antipsychotics are divided into two classes: classic or typical and atypical. The paliperidone, the major metabolite of risperidone, shares with the native drug the characteristics of receptoral bond and of antagonism of serotonin (5HT2A) and dopamine (D2). It's available in a prolonged release formulation and it allows the administration once daily. Besides, the paliperidone has a pharmacological action independent of CYT P450 and in such way a lot of due pharmacological interactions would be avoided to interference with the activity of the CYP2D6, that is known to have involved in the metabolism of the 25% of the drugs of commune therapeutic employment. Introduction The schizophrenia has been a very hard disease to investigate by the research. This is not surprising because it involves the most mysterious aspects of human mind, as emotions and cognitive processes. According to scientific conventions, the schizophrenia is a kind of psychiatric disease, characterized by a course longer than six months (usually chronic or relapsing), by the persistence of symptoms of alteration of mind, behaviour and emotion, with such a seriousness to limitate the normal activity of a person. -

Potential Drug-Drug Interactions and Adverse Drug Reactions Associated with Hydroxychloroquine
Original Article Potential Drug-drug Interactions and Adverse Drug Reactions Associated with Hydroxychloroquine Arjun Singh1, Richa Chaudhary1, Prayas Verma2, Nilanchal Trivedi3,*, Md. Shamim4 1Department of Pharmacy Practice, Teerthanker Mahaveer College of Pharmacy, TMU, Moradabad, Uttar Pradesh, INDIA. 2Teerthanker Mahaveer Dental College and Research Centre, TMU Moradabad, Uttar Pradesh, INDIA. 3Department of Pharmacology, Teerthanker Mahaveer College of Pharmacy, TMU, Moradabad, Uttar Pradesh, INDIA. 4LCP College of Pharmacy Baghpat, Abdul Kalam Technical University (AKTU), Uttar Pradesh, INDIA. ABSTRACT Introduction: COVID-19 is a pandemic disaster and a health emergency of prime focus for all the world economies. Various prophylactic treatments are considered to combat the disease. Hydroxychloroquine drug is one such option that is given much attention as an armor against SARS COV-2 pandemic. Evaluation and assessment of drug interactions and ADRs is required from ethical concern to justify the use of HCQ on such large scale. Methods: We have performed an analysis of HCQ drug interactions on Micromedex®. We have reviewed literature of HCQ pharmacokinetic properties, ADRs/ ADEs and toxicities associated with the use of HCQ drug on PubMed, Google Scholar and CDC database. Results: There are around 180 drug interactions possible with HCQ. Out of them 13 are of contraindicated severity level and other 165 are of major severity and 2 of them are moderately severe. Most of the interactions are coupled with QT prolonging agents (170), Cardiac arrhythmias is possible with the concomitant use of at least 2 drugs, 4 drugs leads to Torsade de points. System organ level ADRs are also evaluated along with various precautions, warnings and contraindications. -

Use of Antipsychotic Drugs and Lithium in Mania
BRITISHJOURNAL OF PSYCHIATRY %20%2001), 01), 178 %suppl. 41), s14s148^ 8^ s156 Use of antipsychotic drugs and lithium in mania experience over the following 30 years indi- cated that lithium is far short of an ideal treatment, even when administered expertly JOHN COOKSON to `concordant' patients. There is a striking difference between the results of early con- trolled trials and the outcome on lithium in open clinical practice, where a smaller pro- portion of patients appear to be `lithium re- sponders'; some even doubt the validity of the clinical trials %Moncrieff, 1997). The benefits of lithium therapy have to be balanced against the difficulties in its Background Studies highlighting the Mania or a manic episode is a severe phase management ± with poor compliance in pa- difficulties associated withlithium suggest in the course of bipolar disorders, the con- tients with bipolar disorder and the occur- dition termed `manic±depressive illness' by rence of withdrawal mania ± and the risks that the role of antipsychotic drugs and Kraepelin in the 19th century. A contem- of side-effects and toxicity %Cookson, mood stabilisersin bipolardisorder should porary textbookdescribed the difficulty of 1997). The latter includes the possibility be reconsidered. managing a patient with mania ± using of permanent neurological sequelae with drugs of limited efficacy, mainly sedative cerebellar damage %Schou, 1984). Aims Toreview the efficacyandmode or anaesthetic, such as chloral and ur- Since the 1970s anticonvulsant drugs, of action of antipsychotic drugsin mania, ethane, or potentially toxic drugs, such as first carbamazepine and more recently and to consider the differences between potassium bromide. The sedative anticon- valproate and, in open studies, lamotrigine officialguidelines and routine clinical vulsant paraldehyde was also used. -

Screening of 300 Drugs in Blood Utilizing Second Generation
Forensic Screening of 300 Drugs in Blood Utilizing Exactive Plus High-Resolution Accurate Mass Spectrometer and ExactFinder Software Kristine Van Natta, Marta Kozak, Xiang He Forensic Toxicology use Only Drugs analyzed Compound Compound Compound Atazanavir Efavirenz Pyrilamine Chlorpropamide Haloperidol Tolbutamide 1-(3-Chlorophenyl)piperazine Des(2-hydroxyethyl)opipramol Pentazocine Atenolol EMDP Quinidine Chlorprothixene Hydrocodone Tramadol 10-hydroxycarbazepine Desalkylflurazepam Perimetazine Atropine Ephedrine Quinine Cilazapril Hydromorphone Trazodone 5-(p-Methylphenyl)-5-phenylhydantoin Desipramine Phenacetin Benperidol Escitalopram Quinupramine Cinchonine Hydroquinine Triazolam 6-Acetylcodeine Desmethylcitalopram Phenazone Benzoylecgonine Esmolol Ranitidine Cinnarizine Hydroxychloroquine Trifluoperazine Bepridil Estazolam Reserpine 6-Monoacetylmorphine Desmethylcitalopram Phencyclidine Cisapride HydroxyItraconazole Trifluperidol Betaxolol Ethyl Loflazepate Risperidone 7(2,3dihydroxypropyl)Theophylline Desmethylclozapine Phenylbutazone Clenbuterol Hydroxyzine Triflupromazine Bezafibrate Ethylamphetamine Ritonavir 7-Aminoclonazepam Desmethyldoxepin Pholcodine Clobazam Ibogaine Trihexyphenidyl Biperiden Etifoxine Ropivacaine 7-Aminoflunitrazepam Desmethylmirtazapine Pimozide Clofibrate Imatinib Trimeprazine Bisoprolol Etodolac Rufinamide 9-hydroxy-risperidone Desmethylnefopam Pindolol Clomethiazole Imipramine Trimetazidine Bromazepam Felbamate Secobarbital Clomipramine Indalpine Trimethoprim Acepromazine Desmethyltramadol Pipamperone -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine -

Stemzine® (Prochlorperazine Maleate) Tablet
AUSTRALIAN PRODUCT INFORMATION – STEMZINE® (PROCHLORPERAZINE MALEATE) TABLET 1 NAME OF THE MEDICINE Prochlorperazine maleate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Stemzine contains 5mg prochlorperazine maleate as the active ingredient. Excipients of known effect: wheat starch (gluten). For the full list of excipients, see Section 6.1 List of excipients. 3 PHARMACEUTICAL FORM Stemzine 5 mg tablets are off-white to pale cream coloured circular tablets, not more than slightly mottled or specked, one side impressed with 'S' and reverse face plain. 4 CLINICAL PARTICULARS 4.1 THERAPEUTIC INDICATIONS Nausea and vomiting due to various causes including migraine; vertigo due to Meniere's syndrome, labyrinthitis and other causes. 4.2 DOSE AND METHOD OF ADMINISTRATION Nausea and vomiting Adults Dosage should be adjusted to suit the response of the individual, beginning with lowest recommended dosage. Oral: 5 or 10 mg two or three times daily. Acute: 20 mg at once, followed, if necessary by 10 mg two hours later. Children (See Section 4.4 Special warnings and precautions for use, Paediatric Use). stem-ccsiv5-piv19-04jun21 Page 1 of 16 If it is considered unavoidable to use prochlorperazine for a child, the dosage is 250 micrograms/kg bodyweight two or three times a day. Prochlorperazine has been associated with dystonic reactions particularly after a cumulative dosage of 500 micrograms/kg. It should therefore be used cautiously in children. Prochlorperazine is not recommended for children weighing less than 10 kg. When treating children, it is recommended that the 5 mg tablets are used. Vertigo and meniere’s disease Adults Oral: 5 to 10 mg three or four times daily. -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Pharmaceuticals and Medical Devices Safety Information No
Pharmaceuticals and Medical Devices Safety Information No. 260 August 2009 Table of Contents 1. Tricyclic and tetracyclic antidepressants, associated with aggression ................................................................................................... 3 2. Important Safety Information .................................................................. 9 .1. Telmisartan ··························································································· 9 .2. Phenytoin, Phenytoin/Phenobarbital, Phenytoin/Phenobarbital/ Caffeine and Sodium Benzoate, Phenytoin Sodium ··························· 13 3. Revision of PRECAUTIONS (No. 208) Lamotrigine (and 9 others) ············································································ 17 4. List of products subject to Early Post-marketing Phase Vigilance ..................................................... 21 This Pharmaceuticals and Medical Devices Safety Information (PMDSI) is issued based on safety information collected by the Ministry of Health, Labour and Welfare. It is intended to facilitate safer use of pharmaceuticals and medical devices by healthcare providers. PMDSI is available on the Pharmaceuticals and Medical Devices Agency website (http://www.pmda.go.jp/english/index.html) and on the MHLW website (http://www.mhlw.go.jp/, Japanese only). Published by Translated by Pharmaceutical and Food Safety Bureau, Pharmaceuticals and Medical Devices Agency Ministry of Health, Labour and Welfare Pharmaceutical and Food Safety Bureau, Office of Safety I, Ministry of -
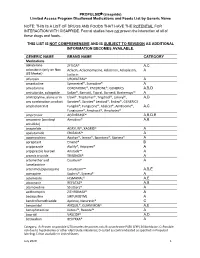
PROPULSID® (Cisapride) Limited Access Program
PROPULSID (cisapride) Limited Access Program Disallowed Medications and Foods List by Generic Name NOTE: THIS IS A LIST OF DRUGS AND FOODS THAT HAVE THE POTENTIAL FOR INTERACTION WITH CISAPRIDE. Formal studies have not proven the interaction of all of these drugs and foods. THIS LIST IS NOT COMPREHENSIVE AND IS SUBJECT TO REVISION AS ADDITIONAL INFORMATION BECOMES AVAILABLE. GENERIC NAME BRAND NAME CATEGORY Medications abiraterone ZYTIGA® A,C aclarubicin (only on Non Aclacin, Aclacinomycine, Aclacinon, Aclaplastin, A US Market) Jaclacin alfuzosin UROXATRAL® A amantadine Symmetrel®, Symadine® A amiodarone CORDARONE®, PACERONE®, GENERICS A,B,D amisulpride, sultopride Solian®, Barnotil, Topral, Barnetil, Barhemsys™ A amitriptyline, alone or in Elavil®, Tryptomer®, Tryptizol®, Laroxyl®, A,D any combination product Saroten®, Sarotex® Lentizol®, Endep®, GENERICS amphotericin B Fungilin®, Fungizone®, Abelcet®, AmBisome®, A,C Fungisome®, Amphocil®, Amphotec® amprenavir AGENERASE® A,B,D,E amsacrine (acridinyl Amsidine® A,B anisidide) anagrelide AGRYLIN®, XAGRID® A apalutamide ERLEADA™ A apomorphine Apokyn®, Ixense®, Spontane®, Uprima® A aprepitant Emend® B aripiprazole Abilify®, Aripiprex® A aripiprazole lauroxil Aristada™ A arsenic trioxide TRISENOX® A artemether and Coartem® A lumefantrine artenimol/piperaquine Eurartesim™ A,B,E asenapine Saphris®, Sycrest® A astemizole HISMANAL® A,E atazanavir REYATAZ® A,B atomoxetine Strattera® A azithromycin ZITHROMAX® A bedaquiline SIRTURO(TM) A bendroflumethiazide Aprinox, Naturetin® C benperidol ANQUIL®, -

Cns Active Principles from Selected Chinese Medicinal Plants
THE FACULTY OF MEDICINE IN THE UNIVERSITY OF LONDON CNS ACTIVE PRINCIPLES FROM SELECTED CHINESE MEDICINAL PLANTS Thesis presented by MIN ZHU (BSc., MSc.) for the degree of Doctor of Philosophy Department of Pharmacognosy The School of Pharmacy University of London 1994 ProQuest Number: 10105154 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest 10105154 Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 ABSTRACT In order to identify potential central nervous system (CNS) active principles from plants, 10 Chinese herbs have been selected from literature reports, namely Schejflera hodinieri, Schejflera delavayi, Celastrus angulatus, Celastrus orbiculatus, Clerodendrum mandarinorum, Clerodendrum bungei, Periploca callophylla, Periploca forrestii, Alangium plantanifolium and Uncaria rhynchophylla. These plants were extracted by 70% ethanol and biologically screened by receptor ligand binding assays which included a 1-adrenoceptor, a2-adrenoceptor, p-adrenoceptor, 5HT1, 5HT1A, 5HT1C, 5HT2, opiate, benzodiazepine, Ca^-ion channel(DHP), K^-ion channel, dopamine 1, dopamine 2, adenosine 1, muscarinic, histamine 1, Na'^/K^ ATPase, GABA^ and GABAg receptors. The results of extract screening showed that all these plants were able to inhibit the specific binding of radioligands to at least one receptor at concentrations of 1 mg/ml. -

Summary of Product Characteristics
NL/H/0662/001/MR NL/H/0663/001/MR NL/H/0664/001/MR SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Tiaprid XXX 100 mg, tablets 2. QUALITATIVE AND QUANTIATIVE COMPOSITION Each tablet contains 100 mg tiapride (as hydrochloride). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet White, round tablets with beveled edge and a cross on both sides. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications For the treatment of neuroleptic-induced tardive dyskinesia, mainly oro-bucco-lingual type. 4.2 Posology and method of administration Adults should take 100-200 mg tiapride three times daily, depending on the severity of the disease and the body weight of the individual . The proposed daily dose for the claimed indication is 300-600 mg tiapride. The effect of treatment may not be apparent until after a period of 4-6 weeks of treatment. Tiaprid XXX tablets should preferably be taken with a little liquid after meals. Tiapride is not intended for treatment in children. Dosage in renal impairment Creatinine clearance: 50-80 ml/min = 75 % of the 10-50 ml/min = 50 % normal less than 10 ml/min = 25 % daily dose 4.3 Contraindications – Hypersensitivity to the active substance or to any of the excipients listed in section 6.1. – Concomitant Prolactin-dependent tumours, e.g. pituitary gland prolactinoma and breast cancer. 1 NL/H/0662/001/MR NL/H/0663/001/MR NL/H/0664/001/MR – Phaeochromocytoma. – Association with levodopa or other dopaminergic medicines (see section 4.5). – Neuroleptic malignant syndrome (see section 4.8).