Electroconvulsive Seizures Regulate Gene Expression of Distinct Neurotrophic Signaling Pathways
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Evidence for Gliadin Antibodies As Causative Agents in Schizophrenia
1 Evidence for gliadin antibodies as causative agents in schizophrenia. C.J.Carter PolygenicPathways, 20 Upper Maze Hill, Saint-Leonard’s on Sea, East Sussex, TN37 0LG [email protected] Tel: 0044 (0)1424 422201 I have no fax Abstract Antibodies to gliadin, a component of gluten, have frequently been reported in schizophrenia patients, and in some cases remission has been noted following the instigation of a gluten free diet. Gliadin is a highly immunogenic protein, and B cell epitopes along its entire immunogenic length are homologous to the products of numerous proteins relevant to schizophrenia (p = 0.012 to 3e-25). These include members of the DISC1 interactome, of glutamate, dopamine and neuregulin signalling networks, and of pathways involved in plasticity, dendritic growth or myelination. Antibodies to gliadin are likely to cross react with these key proteins, as has already been observed with synapsin 1 and calreticulin. Gliadin may thus be a causative agent in schizophrenia, under certain genetic and immunological conditions, producing its effects via antibody mediated knockdown of multiple proteins relevant to the disease process. Because of such homology, an autoimmune response may be sustained by the human antigens that resemble gliadin itself, a scenario supported by many reports of immune activation both in the brain and in lymphocytes in schizophrenia. Gluten free diets and removal of such antibodies may be of therapeutic benefit in certain cases of schizophrenia. 2 Introduction A number of studies from China, Norway, and the USA have reported the presence of gliadin antibodies in schizophrenia 1-5. Gliadin is a component of gluten, intolerance to which is implicated in coeliac disease 6. -
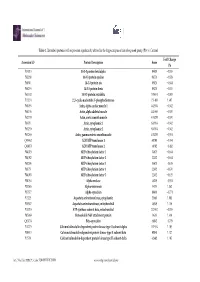
Table 1. Identified Proteins with Expression Significantly Altered in the Hippocampus of Rats of Exposed Group (Pb) Vs
Table 1. Identified proteins with expression significantly altered in the hippocampus of rats of exposed group (Pb) vs. Control. Fold Change Accession Id a Protein Description Score Pb P35213 14-3-3 protein beta/alpha 85420 −0.835 P62260 14-3-3 protein epsilon 96570 −0.878 P68511 14-3-3 protein eta 85420 −0.844 P68255 14-3-3 protein theta 85420 −0.835 P63102 14-3-3 protein zeta/delta 105051 −0.803 P13233 2',3'-cyclic-nucleotide 3'-phosphodiesterase 151400 1.405 P68035 Actin, alpha cardiac muscle 1 442584 −0.942 P68136 Actin, alpha skeletal muscle 441060 −0.970 P62738 Actin, aortic smooth muscle 438270 −0.970 P60711 Actin, cytoplasmic 1 630104 −0.942 P63259 Actin, cytoplasmic 2 630104 −0.942 P63269 Actin, gamma-enteric smooth muscle 438270 −0.951 Q05962 ADP/ATP translocase 1 60100 −0.554 Q09073 ADP/ATP translocase 2 49102 −0.482 P84079 ADP-ribosylation factor 1 34675 −0.644 P84082 ADP-ribosylation factor 2 22412 −0.644 P61206 ADP-ribosylation factor 3 34675 −0.619 P61751 ADP-ribosylation factor 4 22412 −0.670 P84083 ADP-ribosylation factor 5 22412 −0.625 P04764 Alpha-enolase 46219 −0.951 P23565 Alpha-internexin 9478 1.062 P37377 Alpha-synuclein 89619 −0.771 P13221 Aspartate aminotransferase, cytoplasmic 23661 1.083 P00507 Aspartate aminotransferase, mitochondrial 46049 1.116 P10719 ATP synthase subunit beta, mitochondrial 232442 −0.835 P85969 Beta-soluble NSF attachment protein 9638 1.419 Q63754 Beta-synuclein 66842 −0.779 P11275 Calcium/calmodulin-dependent protein kinase type II subunit alpha 181954 1.105 P08413 Calcium/calmodulin-dependent protein kinase type II subunit beta 80840 1.127 P15791 Calcium/calmodulin-dependent protein kinase type II subunit delta 62682 1.105 Int. -

1 Metabolic Dysfunction Is Restricted to the Sciatic Nerve in Experimental
Page 1 of 255 Diabetes Metabolic dysfunction is restricted to the sciatic nerve in experimental diabetic neuropathy Oliver J. Freeman1,2, Richard D. Unwin2,3, Andrew W. Dowsey2,3, Paul Begley2,3, Sumia Ali1, Katherine A. Hollywood2,3, Nitin Rustogi2,3, Rasmus S. Petersen1, Warwick B. Dunn2,3†, Garth J.S. Cooper2,3,4,5* & Natalie J. Gardiner1* 1 Faculty of Life Sciences, University of Manchester, UK 2 Centre for Advanced Discovery and Experimental Therapeutics (CADET), Central Manchester University Hospitals NHS Foundation Trust, Manchester Academic Health Sciences Centre, Manchester, UK 3 Centre for Endocrinology and Diabetes, Institute of Human Development, Faculty of Medical and Human Sciences, University of Manchester, UK 4 School of Biological Sciences, University of Auckland, New Zealand 5 Department of Pharmacology, Medical Sciences Division, University of Oxford, UK † Present address: School of Biosciences, University of Birmingham, UK *Joint corresponding authors: Natalie J. Gardiner and Garth J.S. Cooper Email: [email protected]; [email protected] Address: University of Manchester, AV Hill Building, Oxford Road, Manchester, M13 9PT, United Kingdom Telephone: +44 161 275 5768; +44 161 701 0240 Word count: 4,490 Number of tables: 1, Number of figures: 6 Running title: Metabolic dysfunction in diabetic neuropathy 1 Diabetes Publish Ahead of Print, published online October 15, 2015 Diabetes Page 2 of 255 Abstract High glucose levels in the peripheral nervous system (PNS) have been implicated in the pathogenesis of diabetic neuropathy (DN). However our understanding of the molecular mechanisms which cause the marked distal pathology is incomplete. Here we performed a comprehensive, system-wide analysis of the PNS of a rodent model of DN. -

Mechanisms of Α-Synuclein Induced Synaptopathy in Parkinson’S Disease
King’s Research Portal DOI: 10.3389/fnins.2018.00080 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Bridi, J. C., & Hirth, F. (2018). Mechanisms of -Synuclein Induced Synaptopathy in Parkinson's Disease. Frontiers in Neuroscience, 12, 80. DOI: 10.3389/fnins.2018.00080 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. -

Thesis : Genomic Regulation of Oestrous Behaviour in Dairy Cows
th a ommad K Arun s w o c y r i a in d Arun Kommadath 2012 Propositions 1. Quantitative trait associated gene expression analysis answers different questions than the classical differential expression analysis in microarray based studies (this thesis). 2. Communication between brain areas orchestrates sexual behaviour (this thesis). 3. In bioinformatics analyses, biological interpretation is more important than statistical significance. 4. The trend that technological advances in science outpace legal regulatory frameworks governing them should be reversed. 5. It is from our children that we fully realize what we mean to our parents. 6. In science as in life, what seemed the absolute truth yesterday can be refuted today, and what seems farfetched today can be conventional tomorrow. Propositions belonging to the thesis entitled, ‘Genomic regulation of oestrous behaviour in dairy cows’. Arun Kommadath Wageningen, 24th February 2012 Genomic regulation of oestrous behaviour in dairy cows Thesis committee Thesis supervisor Prof. dr. Mari A. Smits Personal chair at Animal Breeding and Genomics Centre Wageningen University Prof. dr. Martien A.M. Groenen Personal chair at Animal Breeding and Genomics Centre Wageningen University Thesis co-supervisors Dr. Marinus F.W. te Pas Senior Researcher at Animal Breeding and Genomics Centre Wageningen University Other members Prof. dr. Bas Kemp, Wageningen University Prof. dr. Jaap Keijer, Wageningen University Prof. dr. Dirk-Jan de Koning, Swedish University of Agricultural Sciences, Uppsala, Sweden Prof. dr. Eckhard Wolf, Ludwig-Maximilians University, Munich, Germany This research was conducted under the auspices of the Graduate School of Wageningen Institute of Animal Sciences (WIAS). Genomic regulation of oestrous behaviour in dairy cows Arun Kommadath Thesis submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof.dr. -

Data Sheet 106
Rudolf-Wissell-Str. 28a Background 37079 Göttingen, Germany Phone: +49 551-50556-0 Synapsins are neuron-specific phosphoproteins that are exclusively associated with small synaptic Fax: +49 551-50556-384 vesicles, with little or no expression in other tissues including neuroendocrine cells. In mammals, three E-mail: [email protected] distinct synapsin genes (synapsin 1, 2, and 3) encode more than eight neuronal isoforms. Web: www.sysy.com Synapsin 1 is one of the most specific markers of synapses throughout the central and peripheral nervous system. In addition to synaptic nerve terminals, the protein is also present in certain sensory Synapsin 1/2 nerve endings. It is expressed in two splice variants (synapsin 1a and synapsin 1b). Synapsin 1 interacts Cat.No. 106 004; Polyclonal Guinea pig antibody, 100 µl antiserum (lyophilized) with vesicle membranes as well as with actin and spectrin. Synapsin 2 is expressed in the nervous system and also two splice variants were described so far, while synapsin 3 shows a more restricted expression pattern and is mainly found in the hypocampus. Data Sheet Synapsins are major phosphoproteins and are substrates for several protein kinases such as PKA, CaMK I and CaMK II. Synapsin 1 is widely used as reference substrate for calmodulin-dependent protein kinases. Reconstitution/ 100 µl antiserum, lyophilized. For reconstitution add 100 µl H2O, then aliquot and Storage store at -20°C until use. For detailed information, see back of the data sheet. Selected References for 106 004 Applications WB: 1 : 1000 (AP staining) IP: not tested yet An integrated transcriptomics and proteomics analysis reveals functional endocytic dysregulation caused by mutations in ICC: 1 : 1000 LRRK2. -

Identification of RNA Bound to the TDP-43 Ribonucleoprotein Complex in the Adult Mouse Brain
Identification of RNA bound to the TDP-43 ribonucleoprotein complex in the adult mouse brain Running Title: Identification of RNA bound to TDP-43 Ramesh K. Narayanan1, Marie Mangelsdorf1, Ajay Panwar1, Tim J. Butler1, Peter G. Noakes1,2, Robyn H. Wallace1,3* 1Queensland Brain Institute, 2School of Biomedical Sciences, 3School of Chemistry and Molecular Biosciences, The University of Queensland, Brisbane, QLD, 4072, Australia *Corresponding author: [email protected] Objectives. Cytoplasmic inclusions containing TDP-43 are a pathological hallmark of several neurodegenerative disorders, including amyotrophic lateral sclerosis (ALS) and frontotemporal dementia. TDP-43 is an RNA binding protein involved in gene regulation through control of RNA transcription, splicing and transport. However, the function of TDP-43 in the nervous system is largely unknown and its role in the pathogenesis of ALS is unclear. The aim of this study was to identify genes in the central nervous system that are regulated by TDP-43. Methods. RNA-immunoprecipitation with anti-TDP-43 antibody, followed by microarray analysis (RIP- chip), was used to isolate and identify RNA bound to TDP-43 protein from mouse brain. Results. This analysis produced a list of 1,839 potential TDP-43 gene targets, many of which overlap with previous studies and whose functions include RNA processing and synaptic function. Immunohistochemistry demonstrated that the TDP-43 protein could be found at the presynaptic membrane of axon terminals in the neuromuscular junction in mice. Conclusions. The finding that TDP-43 binds to RNA that codes for genes related to synaptic function, together with the localisation of TDP-43 protein at axon terminals, suggest a role for TDP-43 in the transport of synaptic mRNAs into distal processes. -

Comparative Expressed-Sequence-Tag Analysis of Differential Gene Expression Profiles in PC-12 Cells Before and After Nerve Growth Factor Treatment NORMAN H
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 8303-8307, August 1995 Cell Biology Comparative expressed-sequence-tag analysis of differential gene expression profiles in PC-12 cells before and after nerve growth factor treatment NORMAN H. LEE, KEITH G. WEINSTOCK, EWEN F. KIRKNESS, JULIE A. EARLE-HUGHES, REBECCA A. FULDNER, SIMOS MARMAROS, ANNA GLODEK, JEANNINE D. GOCAYNE, MARK D. ADAMS, ANTHONY R. KERLAVAGE, CLAIRE M. FRASER, AND J. CRAIG VENTER The Institute for Genomic Research, 932 Clopper Road, Gaithersburg, MD 20878 Communicated by Martin Rodbell, National Institute of Environmental Health Sciences, Research Triangle Park NC, April 24, 1995 ABSTRACT Nerve growth factor-induced differentiation clones identified as differentially regulated by this approach of adrenal chromaffin PC-12 cells to a neuronal phenotype was confirmed by RNA blot analysis, thereby demonstrating involves alterations in gene expression and represents a model the potential of this technique in gene discovery and differ- system to study neuronal differentiation. We have used the ential gene expression.* expressed-sequence-tag approach to identify "600 differen- tially expressed mRNAs in untreated and nerve growth factor- treated PC-12 cells that encode proteins with diverse struc- EXPERIMENTAL PROCEDURES tural and biochemical functions. Many of these mRNAs en- cDNA Libraries. PC-12 cells (American Type Culture Col- code proteins belonging to cellular pathways not previously lection) were cultured and treated with 2.5S NGF (50 ng/ml) known to be regulated by nerve growth factor. Comparative as described (11). PC-12 cells undergo mitotic arrest and expressed-sequence-tag analysis provides a basis for survey- neuron-like phenotypic expression by day 7 of NGF treatment ing global changes in gene-expression patterns in response to (12); hence, cDNA libraries were constructed from untreated biological signals at an unprecedented scale, is a powerful tool PC-12 cells and PC-12 cells treated with NGF for 9 days. -

Mechanisms of Α-Synuclein Induced Synaptopathy in Parkinson's Disease
King’s Research Portal DOI: 10.3389/fnins.2018.00080 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Bridi, J. C., & Hirth, F. (2018). Mechanisms of -Synuclein Induced Synaptopathy in Parkinson's Disease. Frontiers in Neuroscience, 12, 80. https://doi.org/10.3389/fnins.2018.00080 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. -

UNIVERSITY of CALIFORNIA RIVERSIDE Genetic Removal Of
UNIVERSITY OF CALIFORNIA RIVERSIDE Genetic Removal of Matrix Metalloproteinase 9 Rescues the Symptoms of Fragile X Syndrome in a Mouse Model A Dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Neuroscience by Harpreet Kaur Sidhu December 2013 Dissertation Committee: Dr. Iryna M. Ethell, Co-Chairperson Dr. Douglas W. Ethell, Co-Chairperson Dr. Khaleel Abdulrazak Dr. Michael E. Adams Copyright by Harpreet Kaur Sidhu 2013 The Dissertation of Harpreet Kaur Sidhu is approved: Committee Co-Chairperson Committee Co-Chairperson University of California, Riverside Acknowledgements Dr. Lorraine Dansie (Ph.D, St Jude Children’s Research Hospital) started the project investigating the role of the matrix metalloproteinase 9 in Fragile X syndrome and whose work showing behavioral improvements in the Fmr1 ko mice, together with previous studies, formed the basis of this dissertation. Dr. Peter W. Hickmott (Associate Professor, University of California, Riverside) for his invaluable guidance and patience in teaching me electrophysiology and for time on his rig. He has also contributed to the interpretation of the results and editing of the manuscript. iv Dedication I would like to thank my advisors Drs. Iryna Ethell and Douglas Ethell for all their guidance, patience, direction and support throughout my time in graduate school. They were always encouraging even when I was not hopeful of an experiment working or getting results and our frequent discussions always led to interesting new directions for my project. They always pushed me to think more deeply about my project and their standard of excellence made sure I did everything to the best of my abilities. -

SIV-Mediated Synaptic Dysfunction Is Associated with an Increase in Synapsin Site 1 Phosphorylation and Impaired PP2A Activity
7006 • The Journal of Neuroscience, August 28, 2019 • 39(35):7006–7018 Neurobiology of Disease SIV-Mediated Synaptic Dysfunction Is Associated with an Increase in Synapsin Site 1 Phosphorylation and Impaired PP2A Activity Masoud Shekarabi, Jake A. Robinson, Mandy D. Smith, and Tricia H. Burdo Department of Neuroscience, Comprehensive NeuroAIDS Center, Lewis Katz School of Medicine at Temple University, Philadelphia, Pennsylvania 19140 Although the reduction of viral loads in people with HIV undergoing combination antiretroviral therapy has mitigated AIDS-related symptoms, the prevalence of neurological impairments has remained unchanged. HIV-associated CNS dysfunction includes impair- ments in memory, attention, memory processing, and retrieval. Here, we show a significant site-specific increase in the phosphorylation of Syn I serine 9, site 1, in the frontal cortex lysates and synaptosome preparations of male rhesus macaques infected with simian immunodeficiency virus (SIV) but not in uninfected or SIV-infected antiretroviral therapy animals. Furthermore, we found that a lower protein phosphatase 2A (PP2A) activity, a phosphatase responsible for Syn I (S9) dephosphorylation, is primarily associated with the higher S9 phosphorylation in the frontal cortex of SIV-infected macaques. Comparison of brain sections confirmed higher Syn I (S9) in the frontal cortex and greater coexpression of Syn I and PP2A A subunit, which was observed as perinuclear aggregates in the somata of the frontal cortex of SIV-infected macaques. Synaptosomes from SIV-infected animals were physiologically tested using a synaptic vesicle endocytosis assay and FM4–64 dye showing a significantly higher baseline depolarization levels in synaptosomes of SIV ϩ- infected than uninfected control or antiretroviral therapy animals. -

Anti-Synapsin 2 Antibody (ARG59114)
Product datasheet [email protected] ARG59114 Package: 50 μg anti-Synapsin 2 antibody Store at: -20°C Summary Product Description Rabbit Polyclonal antibody recognizes Synapsin 2 Tested Reactivity Hu, Ms, Rat Tested Application WB Host Rabbit Clonality Polyclonal Isotype IgG Target Name Synapsin 2 Antigen Species Human Immunogen Recombinant protein corresponding to A497-D582 of Human Synapsin 2. Conjugation Un-conjugated Alternate Names Synapsin II; SYNII; Synapsin-2 Application Instructions Application table Application Dilution WB 0.1 - 0.5 µg/ml Application Note * The dilutions indicate recommended starting dilutions and the optimal dilutions or concentrations should be determined by the scientist. Calculated Mw 63 kDa Properties Form Liquid Purification Affinity purification with immunogen. Buffer 0.9% NaCl, 0.2% Na2HPO4, 0.05% Sodium azide and 5% BSA. Preservative 0.05% Sodium azide Stabilizer 5% BSA Concentration 0.5 mg/ml Storage instruction For continuous use, store undiluted antibody at 2-8°C for up to a week. For long-term storage, aliquot and store at -20°C or below. Storage in frost free freezers is not recommended. Avoid repeated freeze/thaw cycles. Suggest spin the vial prior to opening. The antibody solution should be gently mixed before use. Note For laboratory research only, not for drug, diagnostic or other use. www.arigobio.com 1/2 Bioinformation Gene Symbol SYN2 Gene Full Name synapsin II Background This gene is a member of the synapsin gene family. Synapsins encode neuronal phosphoproteins which associate with the cytoplasmic surface of synaptic vesicles. Family members are characterized by common protein domains, and they are implicated in synaptogenesis and the modulation of neurotransmitter release, suggesting a potential role in several neuropsychiatric diseases.