Chapter 6 – State of the Bay, Third Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Texas Rookery Islands Project
5 Chapter 5: Texas Rookery Islands Project 5.1 Restoration and Protection of Texas Rookery Islands: Project Description ................................... 1 5.1.1 Project Summary................................................................................................................. 1 5.1.2 Background and Project Description .................................................................................. 3 5.1.3 Evaluation Criteria ............................................................................................................ 13 5.1.4 Performance Criteria and Monitoring .............................................................................. 14 5.1.5 Offsets ............................................................................................................................... 14 5.1.6 Estimated Cost .................................................................................................................. 15 5.2 Texas Rookery Islands Project: Environmental Assessment ......................................................... 16 5.2.1 Introduction and Background, Purpose and Need ........................................................... 16 5.2.2 Scope of the Environmental Assessment ......................................................................... 17 5.2.3 Project Alternatives .......................................................................................................... 18 5.2.4 Galveston Bay Rookery Islands ........................................................................................ -

Improving Water Quality in Dickinson Bayou One TMDL for Dissolved Oxygen
Texas Commission on Environmental Quality Total Maximum Daily Load Program Improving Water Quality in Dickinson Bayou One TMDL for Dissolved Oxygen Water Quality in Dickinson Bayou Impaired Segment Stream Segment The state of Texas requires that water quality in Project Watershed Houston Seabrook Dickinson Bayou Tidal (Segment 1103) be suitable for Galveston swimming, wading, fishing, and a healthy aquatic HARRIS Bay ecosystem. However, water quality testing found that Webster Kemah dissolved oxygen levels in the water are occasionally low. Oxygen, which dissolves in water, is essential for the survival of aquatic life. While the amount of Friendswood Dickinson dissolved oxygen in water fluctuates naturally, various League City human activities can cause unusually or chronically low 1104 1103 dissolved oxygen levels which may harm fish and other Dickinson Bayou aquatic organisms. Alvin Texas City Santa Fe GALVESTON In response to these conditions, a total maximum daily La Marque load (TMDL) project has been initiated to evaluate the effects of low dissolved oxygen on aquatic life and to BRAZORIA determine the actions necessary to maintain water Hitchcock quality in the tidal portion of Dickinson Bayou. The goal of a TMDL is to determine the amount (or load) of a 012345 pollutant that a body of water can receive and still Miles K support its designated uses. This allowable load is then allocated among all the potential sources of pollution traffic. Rice fields in the upper watershed receive within the watershed. Measures to reduce pollutant irrigation water via canals from beyond the watershed. loads are then developed as necessary. The irrigation water returns to Dickinson Bayou in the form of irrigation return flows. -
Untitled Spreadsheet
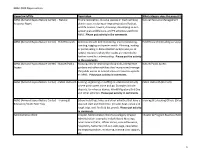
GBAC 2020 Opportunities OpportunityTitle Description What category does the project fall under ABNC (Armand Bayou Nature Center) - Natural Prairie restoration, invasive species or trash removal, Natural Resource Management Resource Mgmt plant rescue, restoring or improving natural habitat, wildlife houses, towers, chimneys, developing an eco- system plan,wildlife care, and P3 activities specific to ABNC. Please put activity in the comments. ABNC (Armand Bayou Nature Center) - Field Research Activities include bird monitoring, insect monitoring, Field Research (including surveys) banding, tagging and species watch. Planning, leading or participating in data collection and/or analysis of natural resources where the results are intended to further scientific understanding. Please put the activity in the comments. ABNC (Armand Bayou Nature Center) - Nature/Public Mowing, new or improving hiking trails, intrepretive Nature/Public Access Access gardens and other activities that improve and manage the public access to natural areas or resources specific to ABNC. Please put activity in comments. ABNC (Armand Bayou Nature Center) - Public Outreach Leading, organizing or staffing an educational activity Public Outreach (Indirect) where participants come and go. Examples include docents, farm house demos, World Migratory Bird Day and other activities. Please put activity in comments. ABNC (Armand Bayou Nature Center) - Training & School Field trips, hikes and other activities that have a Training & Educating Others (Direct) Education/Youth Field Trips planned start and finish time. Includes boat, canoe and kayak trips, owl, firefly & bat prowls. Please put activity in comments. Administrative Work Chapter Administration WorkSub-category Chapter Chapter & Program Business/Administration Administration: examples include Board Meetings, hours administrator, officer duties, committee work, hospitality, Samaritan roll-out, web page, newsletter, training preparation, mentoring, training class support, etc. -

Bastrop Bayou Watershed the Nation’S Leading Rice Ou Can Be Found During Each Season
Understanding the concentrations Bacteria problems in Bastrop LEGEND of bacteria in Bastrop Bayou and Bayou can be exacerbated by failing the potential sources of any septic systems, poorly managed bacteria is very important in land application of sludge from Rice Agriculture assessing risks to the public wastewater treatment plants and Landfill and in preventing water quality reduction in freshwater inflows. has always been a impacts from worsening in Intermittent flow is observed staple of the Brazoria County economy, and rice Great Texas Coastal the future. Elevated bacteria throughout Bastrop Bayou during Bird Trail concentrations are used as dry periods which significantly remains dominant even in indicators of pollution caused by reduce freshwater inflow. Without today’s modern environment. Brazoria National Wildlife nbury human sewage, which can cause the flushing effect from inflow, Farmers in the Bastrop Bayou Da Refuge Boundary serious health concerns like tidal movement remains stagnant watershed started planting rice A us after 1900 and began to dig rice B tin typhoid, cholera and hepatitis. which leads to higher concentrations ay Brazoria National Wildlife Refuge and offers a variety of activities from ou Waterways polluted by human of bacteria. Along with intermittent canals in 1935. From a total of Brazoria National Wildlife Refuge (NWR) is wildlife viewing, photography and sewage can also pose health flow, temperature also plays a 6,000 acres planted in the crop rich in ecology and habitats including biking/biking trails to seasonal events. in 1903, planting grew to ton freshwater sloughs, salt marshes and 16,000 acres by 1940. In Angle Birding and Nature Tourism in Fl native bluestem prairies. -

Stormwater Management Program 2013-2018 Appendix A
Appendix A 2012 Texas Integrated Report - Texas 303(d) List (Category 5) 2012 Texas Integrated Report - Texas 303(d) List (Category 5) As required under Sections 303(d) and 304(a) of the federal Clean Water Act, this list identifies the water bodies in or bordering Texas for which effluent limitations are not stringent enough to implement water quality standards, and for which the associated pollutants are suitable for measurement by maximum daily load. In addition, the TCEQ also develops a schedule identifying Total Maximum Daily Loads (TMDLs) that will be initiated in the next two years for priority impaired waters. Issuance of permits to discharge into 303(d)-listed water bodies is described in the TCEQ regulatory guidance document Procedures to Implement the Texas Surface Water Quality Standards (January 2003, RG-194). Impairments are limited to the geographic area described by the Assessment Unit and identified with a six or seven-digit AU_ID. A TMDL for each impaired parameter will be developed to allocate pollutant loads from contributing sources that affect the parameter of concern in each Assessment Unit. The TMDL will be identified and counted using a six or seven-digit AU_ID. Water Quality permits that are issued before a TMDL is approved will not increase pollutant loading that would contribute to the impairment identified for the Assessment Unit. Explanation of Column Headings SegID and Name: The unique identifier (SegID), segment name, and location of the water body. The SegID may be one of two types of numbers. The first type is a classified segment number (4 digits, e.g., 0218), as defined in Appendix A of the Texas Surface Water Quality Standards (TSWQS). -

Current Status and Historical Trends of Seagrass in the CCBNEP Study
Current Status and Historical Trends of Seagrass in the Corpus Christi Bay National Estuary Program Study Area Corpus Christi Bay National Estuary Program CCBNEP-20 • October 1997 This project has been funded in part by the United States Environmental Protection Agency under assistance agreement #CE-9963-01-2 to the Texas Natural Resource Conservation Commission. The contents of this document do not necessarily represent the views of the United States Environmental Protection Agency or the Texas Natural Resource Conservation Commission, nor do the contents of this document necessarily constitute the views or policy of the Corpus Christi Bay National Estuary Program Management Conference or its members. The information presented is intended to provide background information, including the professional opinion of the authors, for the Management Conference deliberations while drafting official policy in the Comprehensive Conservation and Management Plan (CCMP). The mention of trade names or commercial products does not in any way constitute an endorsement or recommendation for use. Current Status and Historical Trends of Seagrasses in the Corpus Christi Bay National Estuary Program Study Area Warren Pulich, Jr., Ph.D. Catherine Blair Coastal Studies Program Texas Parks & Wildlife Department 3000 IH 35 South Austin, Texas 78704 and William A. White The University of Texas at Austin Bureau of Economic Geology University Station Box X Austin, Texas 78713 Publication CCBNEP - 20 October 1997 Policy Committee Commissioner John Baker Mr. Jerry Clifford Policy Committee Chair Policy Committee Vice-Chair Texas Natural Resource Conservation Acting Regional Administrator, EPA Region 6 Commission The Honorable Vilma Luna Commissioner Ray Clymer State Representative Texas Parks and Wildlife Department The Honorable Carlos Truan Commissioner Garry Mauro Texas Senator Texas General Land Office The Honorable Josephine Miller Commissioner Noe Fernandez County Judge, San Patricio County Texas Water Development Board The Honorable Loyd Neal Mr. -

Houston-Galveston, Texas Managing Coastal Subsidence
HOUSTON-GALVESTON, TEXAS Managing coastal subsidence TEXAS he greater Houston area, possibly more than any other Lake Livingston A N D S metropolitan area in the United States, has been adversely U P L L affected by land subsidence. Extensive subsidence, caused T A S T A mainly by ground-water pumping but also by oil and gas extraction, O C T r has increased the frequency of flooding, caused extensive damage to Subsidence study area i n i t y industrial and transportation infrastructure, motivated major in- R i v vestments in levees, reservoirs, and surface-water distribution facili- e S r D N ties, and caused substantial loss of wetland habitat. Lake Houston A L W O Although regional land subsidence is often subtle and difficult to L detect, there are localities in and near Houston where the effects are Houston quite evident. In this low-lying coastal environment, as much as 10 L Galveston feet of subsidence has shifted the position of the coastline and A Bay T changed the distribution of wetlands and aquatic vegetation. In fact, S A Texas City the San Jacinto Battleground State Historical Park, site of the battle O Galveston that won Texas independence, is now partly submerged. This park, C Gulf of Mexico about 20 miles east of downtown Houston on the shores of Galveston Bay, commemorates the April 21, 1836, victory of Texans 0 20 Miles led by Sam Houston over Mexican forces led by Santa Ana. About 0 20 Kilometers 100 acres of the park are now under water due to subsidence, and A road (below right) that provided access to the San Jacinto Monument was closed due to flood- ing caused by subsidence. -

07-77817-02 Final Report Dickinson Bayou
Dickinson Bayou Watershed Protection Plan February 2009 Dickinson Bayou Watershed Partnership 1 PREPARED IN COOPERATION WITH TEXAS COMMISSION ON ENVIRONMENTAL QUALITY AND U.S. ENVIRONMENTAL PROTECTION AGENCY The preparation of this report was financed though grants from the U.S. Environmental Protection Agency through the Texas Commission on Environmental Quality 2 TABLE OF CONTENTS LIST OF FIGURES ............................................................................................................................................ 7 LIST OF TABLES .............................................................................................................................................. 8 ACKNOWLEDGEMENTS ................................................................................................................................. 9 EXECUTIVE SUMMARY ................................................................................................................................ 10 SUMMARY OF MILESTONES ........................................................................................................................ 13 FORWARD ................................................................................................................................................... 17 1. INTRODUCTION ...................................................................................................................................................... 18 The Dickinson Bayou Watershed .................................................................................................................. -

National Coastal Condition Assessment 2010
You may use the information and images contained in this document for non-commercial, personal, or educational purposes only, provided that you (1) do not modify such information and (2) include proper citation. If material is used for other purposes, you must obtain written permission from the author(s) to use the copyrighted material prior to its use. Reviewed: 7/27/2021 Jenny Wrast Environmental Institute of Houston FY07 FY08 FY09 FY10 FY11 FY12 FY13 Lakes Field Lab, Data Report Research Design Field Lab, Data Rivers Design Field Lab, Data Report Research Design Field Streams Research Design Field Lab, Data Report Research Design Coastal Report Research Design Field Lab, Data Report Research Wetlands Research Research Research Design Field Lab, Data Report 11 sites in: • Sabine Lake • Galveston Bay • Trinity Bay • West Bay • East Bay • Christmas Bay 26 sites in: • East Matagorda Bay • Tres Palacios Bay • Lavaca Bay • Matagorda Bay • Carancahua Bay • Espiritu Santu Bay • San Antonio Bay • Ayres Bay • Mesquite Bay • Copano Bay • Aransas Bay 16 sites in: • Corpus Christi Bay • Nueces Bay • Upper Laguna Madre • Baffin Bay • East Bay • Alazan Bay •Lower Laguna Madre Finding Boat Launches Tracking Forms Locating the “X” Site Pathogen Indicator Enterococcus Habitat Assessment Water Field Measurements Light Attenuation Basic Water Chemistry Chlorophyll Nutrients Sediment Chemistry and Composition •Grain Size • TOC • Metals Sediment boat and equipment cleaned • PCBs after every site. • Organics Benthic Macroinvertebrates Sediment Toxicity Minimum of 3-Liters of sediment required at each site. Croaker Spot Catfish Whole Fish Sand Trout Contaminants Pinfish •Metals •PCBs •Organics Upper Laguna Madre Hurricanes Hermine & Igor Wind & Rain Upper Laguna Madre Copano Bay San Antonio Bay—August Trinity Bay—July Copano Bay—September Jenny Kristen UHCL-EIH Lynne TCEQ Misty Art Crowe Robin Cypher Anne Rogers Other UHCL-EIH Michele Blair Staff Dr. -

Habitat Restoration in the Middle Trinity River Basin When It Comes to Water, the Trinity River Is the Life Blood of People in Dallas/Fort Worth (DFW) and Houston
APRIL 2011 Habitat Restoration in the Middle Trinity River Basin When it comes to water, the Trinity River is the life blood of people in Dallas/Fort Worth (DFW) and Houston. Compromised flow, water quality impairments, and increasing water demands have forced municipalities within the Trinity River Basin to consider long-term solutions for clean water supply often from outside entities (e.g., purchase and transfer of water from other regions of the state). 1 Trinity River—Perspective of History When it comes to water, the Trinity River is the It must have been something to see the Trinity River life blood of people in Dallas/Fort Worth (DFW) prior to European settlement, when Native Americans and Houston. Compromised flow, water quality traveled its bends. One’s imagination can transport impairments, and increasing water demands have you to another time to see the river through the eyes forced municipalities within the Trinity River Basin of French explorer, René Robert La Salle, who stood on to consider long-term solutions for clean water supply its banks in 1687 and was inspired to call it the River often from outside entities (e.g., purchase and transfer of Canoes. of water from other regions of the state). There Rivers were once the highways of frontiersmen, are likely multiple strategies for water supply, but as these waterways afforded the easiest travel, linking maintaining a healthy Trinity River ecosystem is one land with sea and therefore becoming avenues of that is often overlooked. commerce. Over the years as commerce increased, the modest cow town of Fort Worth on the river’s Population Trends and Importance of the northern end combined with neighboring Dallas to Trinity River become one of the top 10 fastest growing metropolitan The population in Texas will expand significantly in areas in the nation. -

Coastal Planning in Texas
Coastal Planning in Texas Ray Newby Coastal Resources Division Texas General Land Office e The Texas General Land Office (GLO) and the Texas Coast • Since 1836: Manager of Tidally-Influenced State-Owned Submerged Lands. • Lead Agency Responsible for: - Coastal Management Program; - Beach and Dune Protection; - State Coastal Erosion Program; - Debris Removal; - Coastal Oil Spill Response; and - Disaster Recovery Program. The Single Deadliest and Three of the Ten Costliest U.S. Hurricanes Impacted Texas #2: $125 Billion – Hurricane Harvey (2017) #7: $34.8 Billion – Hurricane Ike (2008) GLO Coastal Plans • Texas Coastal Resiliency Master Plan provides a framework for community, socio-economic, ecological and infrastructure protection from coastal hazards. The plan is presented to the state legislature. • Coastal Texas Protection & Restoration Feasibility Study, also known as the Coastal Texas Study, is an engineering, environmental and economic analysis to protect the Texas coast. In partnership with the U.S. Army Corps of Engineers, the study will present coastal storm risk management and ecosystem restoration alternatives to Congress for funding. 4 Planning Team for Texas Coastal Resiliency Master Plan • GLO contracted with AECOM for engineering services, the Harte Research Institute for data analysis, and Crouch Environmental for education and outreach. • The Technical Advisory Committee (TAC) is made up of more than 100 coastal experts. 2017 Texas Coastal Resiliency Master Plan • Described the State of the Coast and a Path Forward toward -

Houston a Year After Harvey: Where We Are and Where We Need to Be Presentation by Jim Blackburn Baker Institute and Bayou City Initiative August 30, 2018
Houston A Year After Harvey: Where We Are and Where We Need To Be Presentation By Jim Blackburn Baker Institute and Bayou City Initiative August 30, 2018 Harris County Watersheds Population By Watershed Homes Flooded DuringNumber of Harvey Homes By Watershed Flooded in Hurricane Harvey 26,750 30,000 24,730 25,000 20,000 17,090 14,880 15,000 9,450 12,370 11,980 9,120 7,420 3,790 10,000 6,010 2,200 1,890 510 2,720 5,000 310 1,910 230 190 0 490 0 Percentage of Population with Flooded Homes - Per Watershed 0% 2% 4% 6% 8% 10% 12% Spring Creek Watershed 1% Willow Creek Watershed 1% Addicks Watershed 2% Barker Watershed 2% Luce Bayou Watershed 2% Armand Bayou Watershed 3% Cypress Creek Wshed. (w/ Little Cypr. Crk) 3% Galveston Bay Drainage 3% Vince Bayou Watershed 3% White Oak Bayou Watershed 3% Buffalo Bayou Watershed 4% Brays Bayou Wshed. (w/Willow Waterhole) 4% Spring Gulley & Goose Crk. Watershed 4% Greens Bayou Wshed. (w/Halls Bayou) 5% Sims Bayou Wshed. (w/Berry Bayou) 5% San Jacinto River Wshed. (w/Ship Channel) 5% Cedar Bayou Watershed 6% Clear Creek Watershed (w/Turkey Creek) 7% Hunting Bayou Watershed 10% Percentage of Population with Flooded Homes - Per Watershed 0% 2% 4% 6% 8% 10% 12% Spring Creek Watershed 1% Willow Creek Watershed 1% Addicks Watershed 2% Barker Watershed 2% Luce Bayou Watershed 2% Armand Bayou Watershed 3% Cypress Creek Wshed. (w/ Little Cypr. Crk) 3% Galveston Bay Drainage 3% Vince Bayou Watershed 3% White Oak Bayou Watershed 3% Buffalo Bayou Watershed 4% Brays Bayou Wshed.