1626 Original Article Groundwater Quality Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
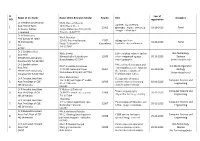
Sl. NO. Name of the Guide Name of the Research Scholar Reg.No Title Year of Registration Discipline 1. Dr.V.Rilbert Janarthanan
Sl. Year of Name of the Guide Name of the Research Scholar Reg.No Title Discipline NO. registration Dr.V.Rilbert Janarthanan Mr.K.Ganesa Moorthy Gjpdz; fPo;f;fzf;F Asst.Prof of Tamil 103D,North Street 1. 11001 Ey;fSk; r*fg; gz;ghl;L 29-10-2013 Tamil St.Xaviers College Arugankulam(po),Sivagiri(tk) khw;Wk; gjpTfSk; Tirunelveli Tirunelveli-627757 Dr.A.Ramasamy Ms.P.Natchiar Prof & HOD of Tamil 22M.K Srteet vallam(po) 11002 vLj;Jiug;gpay; 2. M.S.University 30-10-2013 Tamil Ilangi Tenkasi(tk) (Cancelled) Nehf;fpd; rpyg;gjpf;fhuk; Tvl Tvl-627809 627012 Dr.S.Senthilnathan Mr.E.Edwin Effect of plant extracts and its Bio-Technology Asst.Prof 3. Moonkilvillai Kalpady(po) 11003 active compound against 30-10-2013 Zoology SPKCES M.S.University Kanyakumari-629204 stored grain pest (inter disciplinary) Alwarkurichi Tvl-627412 Dr.S.Senthilnathan Effect of medicinal plant and Mr.P.Vasantha Srinivasan Bio-Medical genetics Asst.Prof entomopatho generic fungi on 4. 11/88 B5 Anjanaya Nagar 11004 30-10-2013 Zoology SPKCES M.S.University the immune response of Suchindram K.K(dist)-629704 (inter disciplinary) Alwarkurichi Tvl-627412 Eepidopternam Larrae Ms.S.Maheshwari Dr.P.Arockia Jansi Rani Recognition of human 1A/18 Bryant Nagar,5th middle Computer Science and 5. Asst.Prof,Dept of CSE 11005 activities from video using 18-11-2013 street Tuticorin Engineering classificaition methods MS University 628008 Dr.P.Arockia Jansi Rani P.Mohamed Fathimal Visual Cryptography Computer Science and 6. Asst.Prof,Dept of CSE 70,MGP sannathi street pettai 11006 20-11-2013 Algorithm for image sharing Engineering MS University Tvl-627004 J.Kavitha Dr.P.Arockia Jansi Rani 2/9 vellakoil suganthalai (po) Combination of Structure and Computer Science and 7. -

Mint Building S.O Chennai TAMIL NADU
pincode officename districtname statename 600001 Flower Bazaar S.O Chennai TAMIL NADU 600001 Chennai G.P.O. Chennai TAMIL NADU 600001 Govt Stanley Hospital S.O Chennai TAMIL NADU 600001 Mannady S.O (Chennai) Chennai TAMIL NADU 600001 Mint Building S.O Chennai TAMIL NADU 600001 Sowcarpet S.O Chennai TAMIL NADU 600002 Anna Road H.O Chennai TAMIL NADU 600002 Chintadripet S.O Chennai TAMIL NADU 600002 Madras Electricity System S.O Chennai TAMIL NADU 600003 Park Town H.O Chennai TAMIL NADU 600003 Edapalayam S.O Chennai TAMIL NADU 600003 Madras Medical College S.O Chennai TAMIL NADU 600003 Ripon Buildings S.O Chennai TAMIL NADU 600004 Mandaveli S.O Chennai TAMIL NADU 600004 Vivekananda College Madras S.O Chennai TAMIL NADU 600004 Mylapore H.O Chennai TAMIL NADU 600005 Tiruvallikkeni S.O Chennai TAMIL NADU 600005 Chepauk S.O Chennai TAMIL NADU 600005 Madras University S.O Chennai TAMIL NADU 600005 Parthasarathy Koil S.O Chennai TAMIL NADU 600006 Greams Road S.O Chennai TAMIL NADU 600006 DPI S.O Chennai TAMIL NADU 600006 Shastri Bhavan S.O Chennai TAMIL NADU 600006 Teynampet West S.O Chennai TAMIL NADU 600007 Vepery S.O Chennai TAMIL NADU 600008 Ethiraj Salai S.O Chennai TAMIL NADU 600008 Egmore S.O Chennai TAMIL NADU 600008 Egmore ND S.O Chennai TAMIL NADU 600009 Fort St George S.O Chennai TAMIL NADU 600010 Kilpauk S.O Chennai TAMIL NADU 600010 Kilpauk Medical College S.O Chennai TAMIL NADU 600011 Perambur S.O Chennai TAMIL NADU 600011 Perambur North S.O Chennai TAMIL NADU 600011 Sembiam S.O Chennai TAMIL NADU 600012 Perambur Barracks S.O Chennai -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2009-11. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2011 [Price: Rs. 27.20 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 33] CHENNAI, WEDNESDAY, AUGUST 31, 2011 Aavani 14, Thiruvalluvar Aandu–2042 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages Change of Names .. 11913-1980 Notices .. 1 — NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES My daughter, A. Mariyapeulla, born on 19th May 2000 I, Y. Jeyanthi Jenet, wife of Thiru C. Yogarajan, born on (native district: Kancheepuram), residing at No. 15, Annaimary 12th February 1973 (native district: Tiruchirappalli), residing 3rd Street, Kamarajapuram, Anakaputtur, Chennai-600 070, at No. 14, Poon Thottam, Manikandan Nagar, Edayarpalayam shall henceforth be known as A. UPAGARANANSI. Main Road, Kuniamuthur, Coimbatore-641 008, shall henceforth be known as Y. JEYANTHI. A. ANDREWS. Chennai, 22nd August 2011. (Father.) Y. JEYANTHI JENET. Coimbatore, 22nd August 2011. I, R. Jothimani, wife of Thiru KA. MAA. Malariniyan, born on 3rd January 1977 (native district: Tiruppur), residing at My daughter, S.M. Dollusubraja, born on 23rd May 1999 Old No. 1, New No. 82/1, V.V. Layout 5th Street, Udumalpet, (native district: Madurai), residing at No. 18-B, Tiruppur-642 126, shall henceforth be known Subramaniapuram 2nd Main Street, Madurai-625 011, shall as VA. -

Tamil Nadu Land Acquisition And
Land Acquisition and Resettlement Due Diligence Report ____________________________________________________________________________ Document Stage: Draft Project Number: 40648-037 February 2018 IND: Infrastructure Development Investment Program for Tamil Nadu (Project 4) - Tranche 4 Appendix 1 - No Objection Certificates of Subprojects (Part 1) Prepared by the Department of Tourism, Government of Tamil Nadu for the Asian Development Bank. This due diligence report is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, management, or staff, and may be preliminary in nature. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. 22 Appendix 1 NO OBJECTION CERTIFICATES OF SUBPROJECTS No. Content Page No. I. Sub Project: IDIPT/TN/P4/T4/ICB/01/2017-18 Supply and Installation of Energy Efficient Lighting (LED) at Major Tourist Cities/ Towns in Tamil Nadu. 1 2 (i). NOC issued by Executive Officer, Arulmigu Papanasaswamy Temple, 27 Papanasam, for installataion of Street Lights in 20 locations 2 2 (ii). NOC issued by Executive Officer, Arulmigu Papanasaswamy Temple, 28 Papanasam, H.R. & C.E. Department for installataion of Street Lights in 20 locations 3 2 (iii). NOC issued by Executive Officer, Arulmigu Papanasaswamy Temple, 29 Papanasam, -

Thoothukudi District
DISTRICT DISASTER MANAGEMENT PLAN 2017 for THOOTHUKUDI DISTRICT on Sendai framework for Disaster Risk Reduction (2015-2030) Index Page Sl.No Chapters No 1 Index / Content of the plan 1-2 2 List of abbreviations present in the plan 3 3 Introduction 4-5 4 District Profile 6-17 5 Disaster Management Goals (2017-30) 18-19 Hazard, Risk and Vulnerability analysis with sample maps & link to all vulnerable maps vulnerability based on 6 a) Infrastructure 20-38 b) Socio – Economic Groups 7 Institutional Mechanism 39-54 8 Preparedness 55-61 9 Prevention & Mitigation Plan (2015-30) 62-87 Response Plan – Including Incident Response System 10 (What Major & Minor Disasters will be addressed through 88-110 mitigation(Covering Rescue,measures) Evacuation , Relief and Industrial Pollution) 11 Recovery and Reconstruction Plan 111-114 Mainstreaming of Disaster Management in Developmental Plans Kudimaramath (PWD) G.O.Ms.No. 50 (Industries Dept – Regarding desilting of tanks) 12 THAI (RD & PR) 115-116 CDRRP MGNREGA Dry land farming ADB – Climate Change Adaptation Scheme IAMWARM etc. Community & other Stakeholder participation CBDRM First Responders 13 NGO‘s 117-122 Red Cross Welfare Associations Local Bodies etc., Linkages / Co-ordination with other agencies for Disaster 14 123-154 Management 1 Budget and Other Financial allocation – Outlays of major 15 155 Schemes Monitoring and Evaluation 16 Hon‘ble Ministers 156-175 Monitoring Officers Inter Departmental Zonal Team (IDZT) Risk Communication strategies 17 176-177 (Telecommunication/VHF/Media/CDRRP etc.) Important Contact numbers and provision for link to detailed 18 178-186 information Do's and Don'ts during all Possible hazards including Heat 19 wave 187-192 20 Important G.Os 193-194 21 Linkages with IDRN 195-240 Specific issues on various Vulnerable Groups have been 22 241-248 addressed 23 Mock Drill Schedules 249 24 Date of approval of DDMP by DDMA 250 2 2. -

State District Branch Address Centre Ifsc Contact1 Contact2 Contact3 Micr Code Andaman and Nicobar Island Andaman Port Blair
STATE DISTRICT BRANCH ADDRESS CENTRE IFSC CONTACT1 CONTACT2 CONTACT3 MICR_CODE FIRST FLOOR, KALAIGNAR ARIVALAYAM, OPP TO Mr.S.Radha DR.B.R.AMBEDKAR krishnan, ANDAMAN AUDITORIUM, 03192- AND MOHANPURA, PORT 239444, portblair@t NICOBAR BLAIR- 744 101, 237222/943 rtgs@tnmbonlin nmbonline. ISLAND ANDAMAN PORT BLAIR ANDAMAN PORT BLAIR TMBL0000323 4298887 e.com com NONMICR Mr.G.Martin Chezhian,M r.S. Muthurajan. Ph.044- 19-8-110, R.C.Road, 26220097,0 ANDHRA Tirupathi-517501 44- PRADESH CHITTOOR TIRUPATHI Tamilnadu TIRUPATI TMBL0000170 26220099 Mr.G.Martin Chezhian,M r.S. Muthurajan. 27-9-50/51, Temple Ph.044- Street, Kakinada- 26220097,0 ANDHRA EAST 533001 Andhra 44- PRADESH GODAVARI KAKINADA Pradesh KAKINADA TMBL0000167 26220099 SURVEY NO.457/1, BESIDE SRI KANYA LODGE, CINEMA ROAD, MANDAPETA- 533 308, EAST Mr.A.Dines GODAVARI h Kumar mandapeta ANDHRA EAST DISTRICT, ANDHRA MANDAPET Ph:917702 rtgs@tnmbonlin @tnmbonli PRADESH GODAVARI MANDAPETA PRADESH A TMBL0000330 9678 e.com ne.com 533060330 Mr.G.Martin Chezhian,M r.S. Muthurajan. 33-1-10 Town Hall Ph.044- Road, Rajahmundry- 26220097,0 ANDHRA EAST 533101 Andhra RAJAHMUN 44- PRADESH GODAVARI RAJAMUNDRY Pradesh DRY TMBL0000120 26220099 GROUND FLOOR, D.NO.28-200, K.B.ROAD, Mr.V.Marim CHILAKALURIPET - uthu Ph: 522 616, GUNTUR 08647- ANDHRA CHILAKALURIPE DISTRICT, ANDHRA CHILAKALU 251065/990 rtgs@tnmbonlin PRADESH GUNTUR T PRADESH RIPET TMBL0000362 8734776 e.com Mr.M.GANE 23-5-7, Santhi building, SAN/0863- ANDHRA Patnam Bazar, Guntur 2322871,22 PRADESH GUNTUR Guntur 522 003 GUNTUR TMBL0000074 22964 D.NO.9-7-40, 9-7-40A AND 9-7-41, 0 ANDHRA ARUNDALPETA, NARSARAO 703272050 PRADESH GUNTUR NARSARAOPET NARASARAOPET PET TMBL0000386 6 D.NO:9 66,GROUND MR.JANAR FLOOR, DHANA PEDANAN P.A.S.COLLEGE RAOMODA DIPADU@ ANDHRA PEDANANDIPAD ROAD, ANDHRA PEDANANDI D,CELL:91 RTGS@TNMBO TNMBONLI PRADESH GUNTUR U PRADESH-522235. -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2017 [Price : Rs. 360.80 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 16C] CHENNAI, WEDNESDAY, APRIL 19, 2017 Chithirai 6, Hevilambi, Thiruvalluvar Aandu–2048 Part VI–Section 1 (Supplement) NOTIFICATIONS BY HEADS OF DEPARTMENTS, ETC. TAMIL NADU NURSES AND MIDWIVES COUNCIL, CHENNAI. (Constituted under Tamil Nadu Act III of 1926) LIST OF NAMES OF NURSE AND MIDWIFE ELECTORAL ROLL FOR THE YEAR 01-01-2012 TO 31-12-2012 DTP—VI-1 Sup. (16C)—1 [ 1 ] THE TAMILNADU NURSES AND MIDWIVES COUNCIL No-140,JeyaPrakash Narayanan Maligai,Santhome High Road, Mylapore,Chennai-600004. emailID:[email protected] LIST OF NAMES OF NURSE AND MIDWIFE ELECTORAL ROLL FOR THE YEAR 01/01/2012 To 31/12/2012 Cat./ Sec. S.No Canditate Name & Address Qualification RNM No. & Reg.Date. 1 Ms MONISHA .N.K B.Sc.,(Nursing) Part :A & C Address:-NELSON .K,MONISHA Section:II COTTAGE,PONVILA,AYIRA POST,, PARASSALA,Thiruvananthapuram , RNM.NO :112374 695502 Date :02/01/2012 2 Ms RADHIKA .R B.Sc.,(Nursing) Part :A & C Address:- Section:I N.RADHAKRISHNAN,2/10,SOUTH STREET,, RNM.NO :112375 P.KADUVETTI ,PADAINILAI Date :02/01/2012 POST,UDAYARPALAYAM,,ARIYALUR, 612903 3 Ms MUTHULAKSHMI .K B.Sc.,(Nursing) Part :A & C Address:-KUNASEKARAN .M,6 Section:I 14.21,CHEKKADI KOIL STREET,, VEERANAM,,TIRUNELVELI, RNM.NO :112376 627861 Date :02/01/2012 4 Ms AMALI H. B.Sc.,(Nursing) Part :A & C Address:-MR. HILLARIS,H. NO;8153 , Section:I KOTTLIPADU,KANNIYAKUMARI, 629251 RNM.NO :112377 Date :02/01/2012 5 Ms KAVITHA K. -

Tamil Nadu Public Service Commission Bulletin
© [Regd. No. TN/CCN-466/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 196/2009 2018 [Price: Rs. 145.60 Paise. TAMIL NADU PUBLIC SERVICE COMMISSION BULLETIN No. 7] CHENNAI, FRIDAY, MARCH 16, 2018 Panguni 2, Hevilambi, Thiruvalluvar Aandu-2049 CONTENTS DEPARTMENTAL TESTS—RESULTS, DECEMBER 2017 NAME OF THE TESTS AND CODE NUMBERS Pages Pages The Tamil Nadu Government office Manual Departmental Test for Junior Assistants In Test (Without Books & With Books) the office of the Administrator - General (Test Code No. 172) 552-624 and official Trustee- Second Paper (Without Books) (Test Code No. 062) 705-706 the Account Test for Executive officers (Without Books& With Books) (Test Code No. 152) 625-693 Local Fund Audit Department Test - Commercial Book - Keeping (Without Books) Survey Departmental Test - Field Surveyor’s (Test Code No. 064) 706-712 Test - Paper -Ii (Without Books) (Test Code No. 032) 694-698 Fisheries Departmental Test - Ii Part - C - Fisheries Technology (Without Books) Fisheries Departmental Test - Ii Part - B - (Test Code No. 067) 712 Inland Fisheries (Without Books) (Test Code No. 060) 698 Forest Department Test - forest Law and forest Revenue (Without Books) Fisheries Departmental Test - Ii Part - (Test Code No. 073) 713-716 A - Marine Fisheries (Without Books) (Test Code No. 054) 699 Departmental Test for Audit Superintendents of Highways Department - Third Paper Departmental Test for Audit Superintendents (Constitution of India) (Without Books) of Highways Department - First Paper (Test Code No. 030) 717 (Precis and Draft) (Without Books) (Test Code No. 020) 699 The Account Test for Public Works Department officers and Subordinates - Part - I (Without Departmental Test for the officers of Books & With Books) (Test Code No. -

Tamil Nadu Siddha Medical Council, Chennai - 106
TAMIL NADU SIDDHA MEDICAL COUNCIL, CHENNAI - 106. REGISTER OF 2D BARCODE ISSUED REGISTERED SIDDHA MEDICAL PRACTITIONERS UPTO 31st MARCH 2017 (Ref No. 60/TNSMC/2017.) A - Class No. VI(1)/330/2017. Sl.No. Reg. No. Name Father's Name Qualification Date of Regn. Address (1) (2) (3) (4) (5) (6) (7) ARIYALUR DISTRICT 1 4608 Dr. AISHWARYA A K. Ambalavanan B.S.M.S. 22/06/2015 No.1334, Palani Andavar Koil Street, Vilandai, Andimadam, Ariyalur - 621801 2 3316 Dr. AKHILA DEVI S P S. Panneer Selvam B.S.M.S. 20/05/2009 I-26-A, Anumar Koil Street, T.Palur, Ariyalur - 612904 M.D.(S) 21/07/2016 3 3993 Dr. AKILA K R. KALIYAPERUMAL B.S.M.S. 24/05/2012 No.1/89, North Street, Sirukalathur Post, Sendurai Taluk, M.D.(S) 14/03/2016 Ariyalur - 621710 4 4693 Dr. AMALA M V. Manickam B.S.M.S. 20/07/2015 No.3/759-3/154, West Street, Mathumadakki, Keezhamaligai Post, Sendurai Taluk, Ariyalur - 621710 5 3903 Dr. ANANTHI K V K. Vaithianathan B.S.M.S. 13/07/2011 No.112, Main Road, Variyankaval, Udayarpalayam Taluk, 2 M.D.(S) 27/01/2015 Ariyalur - 621806 6 4137 Dr. ANBARASI A S. Anbalagan B.S.M.S. 22/07/2013 No.123, Ponparappi / Kudikadu Post, Sendurai Taluk, M.D.(S) 14/03/2017 Ariyalur - 621710 7 4841 Dr. ANILADEVI P S. PANNEER SELVAM B.S.M.S. 07/01/2016 No.1/26, Anumar Koil Street, T. Palur, Ariyalur - 612904 8 4399 Dr. ANITHA A M. -

List of Applications for the Post of Junior Administrative Assistant (Backward Class)
List of applications for the post of Junior Administrative Assistant (Backward Class) Receipt Sl. Whether Application is Accepted (or) Register Name and address of the applicant No. Rejected No. (1) (2) (3) (5) T. Jagatha, D/o. K. Thangappan, 188/93, North Anju Kdueruppu, The applicant is directed to appear before the 1 141 Thengamputhoor P.O., Agasteeswaram Taluk, Kanyakumari District Court, Nagercoil on 07.04.2018 at District. 9.30 A.M for written examination. C.R. Aravind, S/o. M. Chinnakani, 17-34-A, Dharmapuram, The applicant is directed to appear before the 2 145 Eathamozhy Post, Kanyakumari District. District Court, Nagercoil on 07.04.2018 at 9.30 A.M for written examination. The applicant is directed to appear before the N.K. John Davis, S/o. C. Nagandran, 52/23A, Crown Street, 3 148 District Court, Nagercoil on 07.04.2018 at Vathiyarvilai, Krishnancoil, Nagercoil, Kanyakumari District. 9.30 A.M for written examination. T.R. Uthra, D/o. Tamil Selvan, Door No. 31-10.B, Uthra PRiyanga The applicant is directed to appear before the 4 149 Bhavan, Charode, Thuckalay Post, Kanyakumari District, Pin: 629 District Court, Nagercoil on 07.04.2018 at 175. 9.30 A.M for written examination. T.R. Priyanga, D/o. Tamil Selvan, Door No. 31-10.B, Uthra The applicant is directed to appear before the 5 150 PRiyanga Bhavan, Charode, Thuckalay Post, Kanyakumari District, District Court, Nagercoil on 07.04.2018 at Pin: 629 175. 9.30 A.M for written examination. N. Govardhan, S/o. Nagaraj, 20W 3B4/1, Therdral Nager East The applicant is directed to appear before the 6 153 Street, Bodinayakanur P.O., 625 5013, Theni District. -

May Day When Migrant Labourers Walk 1000 Kilometres Back Home
Celebrating May Day when migrant labourers walk 1000 kilometres back home... www.csi1947.com May 2020 COVER BY: Mr. Yesudas P. M. He is a well-known illustration artist for the front line Magazines in Kerala. He is a theatre artist too. Currently, he works as a 2 Drawing Master in a government school in Kerala. COVID 19: A Time to be United, Sharing and EDITOR’S DESK Caring Community reetings in the name of the by certain Dioceses. In the days government in Kerala cohesively Lord Jesus Christ! ahead we also need to provide soaps, handled the pandemic situation is G hand wash solutions and sanitizers appreciable. Likewise, we are called I am writing this message, in large quantity. to continuously uphold the Doctors, while the nations are facing Nurses and other health providers challenges of pandemic Covid-19 The role our mission hospitals has in our regular prayers for their with all its might and expertise. played during this pandemic reminds strength and safety. At this juncture, India has also borne the brunt of me that we are still committed to we express our sincere thanks and the COVID-19. To minimise the carrying out the vision and mission gratitude to our mission partners for spread of the virus, the lockdown of our pioneers who established their prayers and solidarity and we was announced in India from 22nd these hospitals for the welfare of the assure our continuous prayers for March 2020, which has sealed humankind. Stepping-out reminds their wellbeing. borders not only of the states but me of our missionaries who risked of the districts within the states their lives to provide health services, Let us commit ourselves into the and containment area within the while many refrained and refused. -
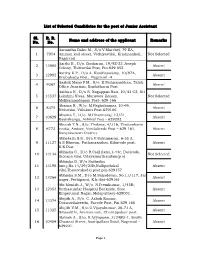
List of Selected Candidates for the Post of Junior Assistant Name And
List of Selected Candidates for the post of Junior Assistant Sl. R. R. Name and address of the applicant Remarks No. No. Aanantha Babu M., S/o.V.Marchel, 79 EA, 1 7954 Amman koil street, Vathiyarvilai, Krishnankoil, Not Selected Nagercoil. Aarthi B., D/o. Baskaran, 19/9Z-23 Joseph 2 13880 Absent Colony, Thittuvilai Post, Pin.629 852. Aarthy K.P., D/o A. Kanthaswamy, 10/97A, 3 12902 Absent Erubukadu Post, Nagercoil -4 Aashik Mano P.M., S/o. K.Padmanabhan, Taluk 4 9267 Absent Office Junction, Kuzhithurai Post. Aathira N., D/o S. Nagappan Nair, 10/43 C2, Sri 5 13537 Lekshmi Nivas, Maruvoor Konam, Not Selected Mekkamandapam Post- 629 166 Abarna R., W/o. M.Reghukumar, 10-49, 6 8375 Absent Neduvilai, Villukuri Post-629180. Abarna T., D/o. M.Dharmaraj, 13/51, 7 10629 Absent Esanthangu, Azhikal Post - 629202. Abeesh T.N., S/o. Thobias, 4/316, Thottankarai 8 6772 veedu, Andoor, Vendalicode Post – 629 161, Absent Kanyakumari District. Abhi-lash S.S., S/o.V.Sukumaran, 6-55 A, 9 11127 A.S.Bhavan, Puthansanthai, Edaicode post, Absent K.K.Dist. Abhisha C., D/o R.Cisil (late),1-44c, Devicode, 10 13134 Not Selected thorana vilai, Udayamarthandam(p.o) Abhisha D.,W/o Sutherlin 11 13198 lino.j,No.14/39/23B,Nullipodukal Absent vilai,Theruvukadai post.pin-629157 Abhisha S.M., D/o M.Sahadevan, No.11/117, Jai 12 13266 Absent nager, Pechiparai, K.k dist-629161 Abi Monish J., W/o. N.Premkumar, 1/45B, 13 12353 Suthasundar Hospital Backside, Sree Absent Emperumal Nagar, Melaputhery-629001.