Cannons of Dindigul Fort
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
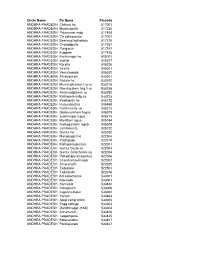
Post Offices
Circle Name Po Name Pincode ANDHRA PRADESH Chittoor ho 517001 ANDHRA PRADESH Madanapalle 517325 ANDHRA PRADESH Palamaner mdg 517408 ANDHRA PRADESH Ctr collectorate 517002 ANDHRA PRADESH Beerangi kothakota 517370 ANDHRA PRADESH Chowdepalle 517257 ANDHRA PRADESH Punganur 517247 ANDHRA PRADESH Kuppam 517425 ANDHRA PRADESH Karimnagar ho 505001 ANDHRA PRADESH Jagtial 505327 ANDHRA PRADESH Koratla 505326 ANDHRA PRADESH Sirsilla 505301 ANDHRA PRADESH Vemulawada 505302 ANDHRA PRADESH Amalapuram 533201 ANDHRA PRADESH Razole ho 533242 ANDHRA PRADESH Mummidivaram lsg so 533216 ANDHRA PRADESH Ravulapalem hsg ii so 533238 ANDHRA PRADESH Antarvedipalem so 533252 ANDHRA PRADESH Kothapeta mdg so 533223 ANDHRA PRADESH Peddapalli ho 505172 ANDHRA PRADESH Huzurabad ho 505468 ANDHRA PRADESH Fertilizercity so 505210 ANDHRA PRADESH Godavarikhani hsgso 505209 ANDHRA PRADESH Jyothinagar lsgso 505215 ANDHRA PRADESH Manthani lsgso 505184 ANDHRA PRADESH Ramagundam lsgso 505208 ANDHRA PRADESH Jammikunta 505122 ANDHRA PRADESH Guntur ho 522002 ANDHRA PRADESH Mangalagiri ho 522503 ANDHRA PRADESH Prathipadu 522019 ANDHRA PRADESH Kothapeta(guntur) 522001 ANDHRA PRADESH Guntur bazar so 522003 ANDHRA PRADESH Guntur collectorate so 522004 ANDHRA PRADESH Pattabhipuram(guntur) 522006 ANDHRA PRADESH Chandramoulinagar 522007 ANDHRA PRADESH Amaravathi 522020 ANDHRA PRADESH Tadepalle 522501 ANDHRA PRADESH Tadikonda 522236 ANDHRA PRADESH Kd-collectorate 533001 ANDHRA PRADESH Kakinada 533001 ANDHRA PRADESH Samalkot 533440 ANDHRA PRADESH Indrapalem 533006 ANDHRA PRADESH Jagannaickpur -

Cadenza Document
ROTARACT AROUND THE WORLD AS OF 23-August-2016 Overall Totals Rotaract Clubs 9,578 Rotary Club Sponsors 8,968 Districts 527 Countries/Geographic Areas 176 World Rotaract Club Growth Rate 0.86% Overall Membership Totals Estimated Membership 220,294 Reported Membership 89,828 Worldwide Reported Membership Growth Rate 1.11% Afghanistan Number of active Rotaract clubs: 1 Change in # of Rotaract clubs since the end of the previous quarter: 1 Number of reported Rotaractors: 1 President /Advisor Total Reported President/Advisor Confirmed District Rotaract Club ID Rotaract Club Name Sponsor Rotary Clubs Term Reported Members Membership Roster 2430 91955 Jalalabad 2014 - 2015 Jalalabad 1 N Data as of: 23-Aug-2016 Page 1 of 661 Rotaract Around the World CC00036.1601 ROTARACT AROUND THE WORLD AS OF 23-August-2016 Albania Number of active Rotaract clubs: 2 Change in # of Rotaract clubs since the end of the previous quarter: 0 Number of reported Rotaractors: 29 President /Advisor Total Reported President/Advisor Confirmed District Rotaract Club ID Rotaract Club Name Sponsor Rotary Clubs Term Reported Members Membership Roster 55 91161 Tirana 2015 - 2016 Tiranë 13 N 55 91821 Tirana Action 2015 - 2016 Tiranë 16 N Data as of: 23-Aug-2016 Page 2 of 661 Rotaract Around the World CC00036.1601 ROTARACT AROUND THE WORLD AS OF 23-August-2016 Algeria Number of active Rotaract clubs: 14 Change in # of Rotaract clubs since the end of the previous quarter: 0 Number of reported Rotaractors: 72 President /Advisor Total Reported President/Advisor Confirmed District -

Annexure – 1 List of Tourist Places in Tamil Nadu -..::Tamilnadu Tourism
Annexure – 1 List of Tourist Places in Tamil Nadu Name of Beaches Eco- Tourism Wildlife / Bird Others Art & Culture / Heritage Pilgrim Centers Hills the District (1) (2) Sanctuary (4 & 5) (6) Stations ( 3) Chennai 1.Elliots Beach 1.Guindy, 1.High Court of 1.St. George Fort 1. AshtalakshmiTemple, 2. Marina Beach Children’s Park Madras 2. Ameer Mahal Chennai2.KapaleeswararTemple, 3. Light House 2.SnakePark 2.Madras University 3. VivekanandarIllam Mylapore 3.Parthasarathi Temple, 3.Rippon Building 4.Valluvar Kottam Triplicane 4. TidelPark 5.Gandhi Mandapam 4.Vadapalani Murugan Temple 5.BirlaKolarangam 6.Kamarajar Memorial 5.St.Andru’s Church 6.Lait Kala Academy 7.M.G.R Memorial 6.Santhome Catherdral 7. AnnanagarTower 8.Periyar Memorial 7.Makka Mosque, Thousand Lights 8.Apollo Hospital 9.Connemara public library 8.Shirdi SaibabaTemple, Mylapore 9.SankaraNethralaya 10.Govt. Museum, Egmore 9.KalingambalTemple, Parry’s 10. Adayar cancer 11.Fort Museum 10.Marundeeswarar Temple, Hospital and 12. Kalashethra Tiruvanmiyur Institute 13. Rail Museum, Perambur 11.Jain Temple 11. Vijaya Hospital, 14. Rajaji Hall 12.Iyyappan Vadaplani 15.Anna Square Temple,Mahalingapuram&Annanagar 12.Sankara 16.Barathiyar Memorial 13.Thirumalai TirupattyDevasthanam, NethralayaEye 17. M.G.R. Illam T. Nagar Hospital. 18. Govt. Fine Arts Collage. 14.Buddhavihar, Egmore 13. Adyar 15.Madhiya Kailash Temple, Adyar BaniyanTree 16.RamakrishnaTemple 14. Arvind Eye 17. Velankanni Church, Beasant Nagar Hospital 18.St. George Catherdral 19. BigMosque,Triplicane. Name of Beaches Eco- Tourism Wildlife / Bird Others Art & Culture / Heritage Pilgrim Centers Hills the District Sanctuary Stations Ariyalur 1.Karaivetti 1.Fossile Museum 1.JayankondamPalace 1.Adaikala Madha Shrine, Elakurichi Bird Sanctuary 2. -

10Th-Std-Social-2Nd-Volume-Book
General Studies Prepared By www.winmeen.com 10th Social 2nd Volume Book Back Questions History Unit 6 - Early Revolts against British Rule in Tamil Nadu Unit 7 - Anti-Colonial Movements and the Birth of Nationalism Unit 8 - Nationalism: Gandhian Phase Unit 9 - Freedom Struggle in Tamil Nadu Unit 10 - Social Transformation in Tamil Nadu Geography Unit 6 - Physical Geography of Tamil Nadu Unit 7 - Human Geography of Tamil Nadu Civics Unit 4 - India’s Foreign Policy Unit 5 - India’s International Relations Economics Unit 3 - Food Security and Nutrition Unit 4 - Government and Taxes Unit 5 - Industrial Clusters in Tamil Nadu Learning Leads To Ruling Page 1 of 103 General Studies Prepared By www.winmeen.com HISTORY Unit - 6 Early revolts against British rule in Tamil Nadu Choose the Correct Answer: 1. Who was the first Palayakkarars to resist the East India Company’s policy of territorial aggrandizement? (a) Marudhu brothers (b) Puli Thevar (c) Velunachiyar (d) Veerapandya Kattabomman 2. Who had borrowed money from the East India Company to meet the expenses he had incurred during the Carnatic wars? (a) Velunachiyar (b) Puli Thevar (c) Nawab to Arcot (d) Raja of Travancore 3. Who had established close relationship with the three agents of Chanda Sahib? (a) Velunachiyar (b) Kattabomman (c) Puli Thevar (d) Oomai Thurai 4. Where was Sivasubramanianar executed? (a) Kayathar (b) Nagalapuram (c) Virupachi (d) Panchalamkurichi 5. Who issued the Tiruchirappalli proclamation of Independence? (a) Marudhu brothers (b) Puli Thevar (c) Veerapandya Kattabomman (d) Gopala Nayak 6. When did the Vellore Revolt breakout? (a) 24 May 1805 (b) 10 July 1805 (c) 10 July 1806 (d) 10 September 1806 7. -

Mint Building S.O Chennai TAMIL NADU
pincode officename districtname statename 600001 Flower Bazaar S.O Chennai TAMIL NADU 600001 Chennai G.P.O. Chennai TAMIL NADU 600001 Govt Stanley Hospital S.O Chennai TAMIL NADU 600001 Mannady S.O (Chennai) Chennai TAMIL NADU 600001 Mint Building S.O Chennai TAMIL NADU 600001 Sowcarpet S.O Chennai TAMIL NADU 600002 Anna Road H.O Chennai TAMIL NADU 600002 Chintadripet S.O Chennai TAMIL NADU 600002 Madras Electricity System S.O Chennai TAMIL NADU 600003 Park Town H.O Chennai TAMIL NADU 600003 Edapalayam S.O Chennai TAMIL NADU 600003 Madras Medical College S.O Chennai TAMIL NADU 600003 Ripon Buildings S.O Chennai TAMIL NADU 600004 Mandaveli S.O Chennai TAMIL NADU 600004 Vivekananda College Madras S.O Chennai TAMIL NADU 600004 Mylapore H.O Chennai TAMIL NADU 600005 Tiruvallikkeni S.O Chennai TAMIL NADU 600005 Chepauk S.O Chennai TAMIL NADU 600005 Madras University S.O Chennai TAMIL NADU 600005 Parthasarathy Koil S.O Chennai TAMIL NADU 600006 Greams Road S.O Chennai TAMIL NADU 600006 DPI S.O Chennai TAMIL NADU 600006 Shastri Bhavan S.O Chennai TAMIL NADU 600006 Teynampet West S.O Chennai TAMIL NADU 600007 Vepery S.O Chennai TAMIL NADU 600008 Ethiraj Salai S.O Chennai TAMIL NADU 600008 Egmore S.O Chennai TAMIL NADU 600008 Egmore ND S.O Chennai TAMIL NADU 600009 Fort St George S.O Chennai TAMIL NADU 600010 Kilpauk S.O Chennai TAMIL NADU 600010 Kilpauk Medical College S.O Chennai TAMIL NADU 600011 Perambur S.O Chennai TAMIL NADU 600011 Perambur North S.O Chennai TAMIL NADU 600011 Sembiam S.O Chennai TAMIL NADU 600012 Perambur Barracks S.O Chennai -
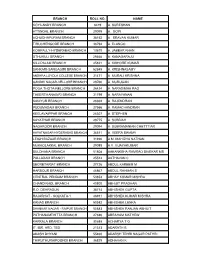
Branch Roll No. Name Koyilandy Branch 6419 A, Sureshan Attingal Branch 29099 A
BRANCH ROLL NO. NAME KOYILANDY BRANCH 6419 A, SURESHAN ATTINGAL BRANCH 29099 A. GOPI ACHUDHAPURAM BRANCH 38152 A. SRAVAN KUMAR TIRUCHENGODE BRANCH 36794 A. ELANGO KOMPALLY-HYDERABAD BRANCH 13670 A. JABBAR KHAN UTHUKULI BRANCH 29466 A. KANAGARAJU KILLIPALAM BRANCH 45821 A. KISHORE KUMAR SANKARI-SANGAGIRI BRANCH 52849 A. KRISHNASAMY ANDHRA LOYOLA COLLEGE BRANCH 31471 A. MURALI KRISHNA GANDHI NAGAR-VELLORE BRANCH 29766 A. MURUGAN POGA THOTA-NELLORE BRANCH 26424 A. NARASIMHA RAO THEERTHANAGIRI BRANCH 31799 A. NARAYANAN KANIYUR BRANCH 28302 A. RAJENDRAN PUDUMADAM BRANCH 27566 A. RAMACHANDRAN KEELAVAIPPAR BRANCH 26307 A. STEPHEN KAYATHAR BRANCH 29775 A. SUBBIAH NAGAROOR BRANCH 29094 A. SUBRAMANIAN CHETTTIAR HAYATNAGAR-HYDERABAD BRANCH 36511 A. VEERA SWAMY LEIGH BAZAAR BRANCH 31926 A.M. MAHIZHA NATHAN MUKKOLAKKAL BRANCH 29088 A.V. VIJAYAKUMAR BULDHANA BRANCH 51808 AAKANKSHA RAMRAO DIVEKAR MS PALLADAM BRANCH 25514 AATHAVAN C SECRETARIAT BRANCH 27726 ABDUL KAREEM M HARBOUR BRANCH 46867 ABDUL RAHMAN S CENTRAL PENDAM BRANCH 53853 ABHAY KUMAR MISHRA CHANDIKHOL BRANCH 40800 ABHIJIT PRADHAN R.O. DEHRADUN 38714 ABHISHEK GUPTA RAJARHAT - KOLKATA-1 46911 ABHISHEK KUMAR MISHRA KANAS BRANCH 50582 ABHISHEK LENKA SHANKAR NAGAR - RAIPUR BRANCH 50883 ABHISHEK RANJAN ABHIJIT PATHANAMTHITTA BRANCH 27846 ABRAHAM MATHEW KARKALA BRANCH 30688 ACHARYA T G IT, IBR, ARD, TBD 21313 ADARATH B AKASH DHYANI 55460 ADARSH TEHRI NAGAR PATHRI THIRUTHURAIPOONDI BRANCH 36829 ADHAVAN K CHINTADRIPET BRANCH 25915 ADHIMOOLAM N C.B.O. LUCKNOW 39922 AGASTYA KUMAR SINGH PANCHUKULA BRANCH -

Indian Overseas Bank
. Indian Overseas Bank REQUEST FOR PROPOSAL FOR SUPPLY, INSTALLATION AND MAINTENANCE OF SELF SERVING PASSBOOK PRINTING KIOSKS ACROSS VARIOUS BRANCHES OF THE BANK RFP REFERENCE NUMBER: RFP/ITD/008/21-22 DATED 23.07.2021 INFORMATION TECHNOLOGY DEPARTMENT (ISO/IEC 27001:2013 CERTIFIED) CENTRAL OFFICE INDIAN OVERSEAS BANK 763, ANNA SALAI ANNEXURE BUILDING CHENNAI 600002 Page 0 of 132 RFP Ref No. RFP/ITD/008/21-22 DATED 23.07.2021 FOR SUPPLY, INSTALLATION AND MAINTENANCE OF SELF SERVING PASSBOOK PRINTING KIOSKS ACROSS VARIOUS BRANCHES OF THE BANK \ INDEX INTRODUCTION .............................................................................................................................................................................. 3 DISCLAIMER .................................................................................................................................................................................... 3 PART I - PROJECT SPECIFIC TERMS & CONDITIONS: .............................................................................................................. 4 1.1 SCHEDULE OF BIDDING PROCESS: ...................................................................................................................... 4 1.2. COST OF BID DOCUMENT& EARNEST MONEY DEPOSITS (EMD): ................................................................. 5 1.3. BIDDER QUALIFICATION CRITIERIA (BQC): ...................................................................................................... 5 1.4 BRIEF REQUIREMENT: ............................................................................................................................................. -

Term Iii Lesson – 1 I. Choose the Correct Answer; 1
NEW BHARATH MATRICULATION HIGHER SECONDARY SCHOOL,TVR SOCIAL KEY ANSWER – TERM III LESSON – 1 I. CHOOSE THE CORRECT ANSWER; 1. Vellore fort was built by Kings of Vijayanagar. a. Udayagiri b. Vellore c. Gingee 2. Thirumalai Nayakhar Palace is situated in Madurai. a. Salem b. Thirumalai c. Madurai 3. Saraswathi Mahal is considered as one of the oldest historical libraries in India. a. Sarawathi b. Lakshmi c. Durga 4. Padmanabhapuram Palace is in Kanyakumari . a. Ooty b. Kanyakumari c. Chennai 5. T harangambadi fort is locally called Danish fort. a. Dindigul b. Gingee c. Thatangambadi II. MATCH THE FOLLOWING; 1. Gingee fort - Villupuram 2. Danish fort - Tharangambadi 3. Tammukkam Palace - Madurai 4. Thirumayam fort - Pudukkottai 5. Fort St., George - Chennai III. TRUE OR FALSE; 1. Tamil Nadu has been ruled by many Rulers. Especially by the Chera,Chola, Pandiya and Pallava rulers. - TRUE 2. Vellore Fort has five mahals. - TRUE 3. Dindigul Fort was built by the Nayaks Madurai. - TRUE 4. Oomayan kottai is the other name for Gingee fort. – FALSE 5. The Padmanabhapuram Palace was built by the ruler of Travancore – TRUE IV. ANSWER THE FOLLOWING; 1. What are the prime attractions of Tamil Nadu tourism? • The architectural monuments are now preserved in the form of palaces, forts and other historical sites. • They are the prime attractions of Tamil Nadu tourism. 2. Write a short note on Tharangambadi fort. • It is located in Tharangambadi of Tamil Nadu. • The fort is trapezoidal in shape. • The central part of the fort has four camels hump shaped domes. • The central pillar of the hall holds the entire weight of the domes. -

List of Potential Sites for Adoption Region: Southern Zone
List of Potential Sites for Adoption Region: Southern Zone State SN Site Images Relevance of the site Karnataka 1. Daria Daulat Bagh, Tipu Sultan built this palace in 1784 and ruled Mysore from Srirangapatnam, Bangalore here for a short time, in the middle of the 18th century. The palace is built in the Indo-Sarcenic style in mostly made of teakwood. 2. Jaina & Vaishna Caves, The Badami cave temples are a complex of four Hindu, a Jain Badami and possibly Buddhist cave temples located in Badami 3. Group of Monuments, A complex of 7th and 8th century CE Hindu and Jain temples in Pattakadal northern Karnataka (India). UNESCO has described Pattadakal as "a harmonious blend of architectural forms from northern and southern India" and an illustration of "eclectic art" at its height. 4. Nandi Hills Fort, There are many stories about the origin of the name Nandi Hills. Chikkaballapur During Chola period, Nandi Hills was called Ananda Giri meaning The Hill of Happiness. Nandi is also commonly called Nandidurga (Fort) because of the fort built here by the ruler Tipu Sultan. Andhra Pradesh 5. Hill Fort, Madakasira The Hill Fort in the village is one of the centrally protected monuments of national importance. In Madakasira you can find a very big hill with a fort and a temple on its top build by Vijayanagara Samrajam. Page | 1 6. Group of 8 Rock-cut There are eight rock cut cave temples having resemblance with Temples at Bhairavakona, Mamallapuram rock cut cave temples. They are located on the Kotapalli side of a granite cliff comprising carved architectural elements such as decorative pillars and finely sculpted panels. -

February 2021 6 1 Web.Indd 1 03-02-2021 11:57:16 Serve to Change Lives Shekhar Mehta’S Presidential Theme
FFebruaryebruary 20212021 @@NewsRotaryNewsRotary //RotaryNewsIndiaRotaryNewsIndia 420 ` ͮŶŶƵĂů^ƵďƐĐƌŝƉƟŽŶ Vol.64, Issue 8 Vol.64, P_Wrapper_Front_Front_Inside_February 2021_6_1_web.indd 1 03-02-2021 11:57:16 Serve to Change Lives Shekhar Mehta’s Presidential theme P_Wrapper_Front_Front_Inside_February 2021_6_1_web.indd 2 03-02-2021 11:57:13 Inside 10 10 Time to upscale our performance RIPE Shekhar Mehta urges incoming district governors to think big, addressing The Odyssey, the 14 virtual Rotary institute. 14 Let us embrace the digital age RI President Holger Knaack tells DGEs that Rotarians should make increasing use of technological tools in his convocation address at GETS. 20 Pandemics offer hope too Trustee chair K R Ravindran explains how TRF 20 responded quickly to help communities during the pandemic. 22 Crucial public healthcare lessons from the Covid pandemic WHO chief scientist Sowmya Swaminathan highlights the need to invest in public health to prevent and control diseases. 24 Our diversity allows us to be so brilliant & shine brightly RIPN Jennifer Jones stresses on the mantra of unity, equity and diversity at two virtual meets held by Synergy Alliance and RC Madras. 22 51 All clubs should get PAN and be KYC compliant RIDs Bharat Pandya and Kamal Sanghvi urge Rotary clubs in India to comply with GoI’s new rules for the voluntary sector. 64 Ravishankar, sitarist nonpareil Read about the life and work of the legendary sitarist. 70 Of shikaras and heaven on earth 24 A nostalgic journey to the all-time favourite TV serial, Gul Gulshan Gulfam, made into a book with the same title. On the cover: A dance by Mansi Karani and troupe as part of Odyssey, the virtual Rotary institute. -

Final Report on 20 Years Perspective Tourism Plan for the State of Tamil Nadu
GOVERNMENT OF INDIA MINISTRY OF TOURISM AND CULTURE DEPARTMENT OF TOURISM MARKET RESEARCH DIVISION FINAL REPORT ON 20 YEARS PERSPECTIVE TOURISM PLAN FOR THE STATE OF TAMIL NADU March 2003 CONSULTING ENGINEERING SERVICES (I) PVT. LTD. NEW DELHI KOLKATA MUMBAI CHENNAI Contents Chapters Page No. Executive Summary 1. Introduction 1.1 Scope of work 1 of 7 1.2 Objectives 3 of 7 1.3 Deliverables 4 of 7 1.4 Approach 4 of 7 1.5 Methodology 6 of 7 1.6 Structure of the Report 7 of 7 2. Profile of the State 2.1 Evolution of Tamil Nadu 1 of 27 2.2 Geographic Features 4 of 27 2.3 Climate 6 of 27 2.4 Econo-Cultural Activities 6 of 27 2.5 Tourism Scenario 15 of 27 2.6 Eco-Tourism in India 18 of 27 2.7 Tourism Policy 27 of 27 3. Tourist Destinations in the State 3.1 Pilgrimage Destinations 2 of 41 3.2 Heritage Locations and Historic Monuments 9 of 41 3.3 Destinations of Scenic Beauty, Forests and Sanctuaries 16 of 41 3.4 Tourist Festival Locations 30 of 41 3.5 Adventure Destinations 34 of 41 3.6 Leisure Destinations 39 of 41 4. Analysis and Forecast 4.1 Trends in Tourist Flow 1 of 27 4.2 SWOT Analysis 7 of 27 4.3 Tourist Forecast 19 of 27 4.4 Potential Destinations 25 of 27 5. Tourism Infrastructure 5.1 Carrying Capacity 2 of 25 5.2 Quality of Service 17 of 25 6. Strengthening Tourism 6.1 WTO’s Bali Declaration 1 of 24 6.2 Strategy for Tourism Promotion 5 of 24 6.3 Product Development 7 of 24 6.4 Augmentation of Infrastructure 17 of 24 6.5 Employment Potential 22 of 24 7. -

Class: V Subject: Social Science I. Choose the Correct Answer. II
UNIT: 1- FORTS AND PALACES Class: V Subject: Social Science I. Choose the correct answer. 1. __________ fort was built by Kings of Vijayanagar. a) Udayagiri b) Vellore c) Gingee 2. Thirumalai Nayakkar Palace is situated in __________ . a) Salem b) Thirumalai c) Madurai 3. _________ Mahal is considered as one of the oldest histroical libraries in India. a) Saraswathi b) Lakshmi c) Durga 4. Padmanabhapuram Palace is in __________. a) Ooty b) Kanyakumari c) Chennai 5. _________fort is locally called Danish fort. a) Dindigul b) Gingee c) Tharangambadi 120 II. Match the following. 1. Gingee fort - Villupuram 2. Danish fort - Tharangambadi 3.Tammukkam Palace - Madurai 4.Thirumayam fort - Pudukottai 5. Fort St. George - Chennai III. True or False. 1. Tamil Nadu has been ruled by many Rulers. Especially by the Chera, Chola, Pandya and Pallava rulers. ( True) 2. Vellore Fort has five mahals. ( True) 3. Dindigul Fort was built by the Nayaks of Madurai. ( True) 4. Oomayan Kottai is the other name for Gingee fort. ( False) 5.The Padmanabhapuram Palace was built by the ruler of Travancore. (True ) IV. Answer the following. 1. What are the prime attractions of Tamil Nadu tourism? Palaces and forts are the prime attractions of Tamil Nadu tourism. 2. Write a short note on Tharangambadi fort. Tharangambadi fort is locally called Danish fort. It is located on the shores of Bay of Bengal in Tharangambadi (Tranquebar), Tamil Nadu. The fort is trapezoidal in shape with three rooms in the left wing. The central pillar of the hall holds the entire weight of the domes.