Update to the Phase I Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lady Crabs, Ovalipes Ocellatus, in the Gulf of Maine
18_04049_CRABnotes.qxd 6/5/07 8:16 PM Page 106 Notes Lady Crabs, Ovalipes ocellatus, in the Gulf of Maine J. C. A. BURCHSTED1 and FRED BURCHSTED2 1 Department of Biology, Salem State College, Salem, Massachusetts 01970 USA 2 Research Services, Widener Library, Harvard University, Cambridge, Massachusetts 02138 USA Burchsted, J. C. A., and Fred Burchsted. 2006. Lady Crabs, Ovalipes ocellatus, in the Gulf of Maine. Canadian Field-Naturalist 120(1): 106-108. The Lady Crab (Ovalipes ocellatus), mainly found south of Cape Cod and in the southern Gulf of St. Lawrence, is reported from an ocean beach on the north shore of Massachusetts Bay (42°28'60"N, 70°46'20"W) in the Gulf of Maine. All previ- ously known Gulf of Maine populations north of Cape Cod Bay are estuarine and thought to be relicts of a continuous range during the Hypsithermal. The population reported here is likely a recent local habitat expansion. Key Words: Lady Crab, Ovalipes ocellatus, Gulf of Maine, distribution. The Lady Crab (Ovalipes ocellatus) is a common flats (Larsen and Doggett 1991). Lady Crabs were member of the sand beach fauna south of Cape Cod. not found in intensive local studies of western Cape Like many other members of the Virginian faunal Cod Bay (Davis and McGrath 1984) or Ipswich Bay province (between Cape Cod and Cape Hatteras), it (Dexter 1944). has a disjunct population in the southern Gulf of St. Berrick (1986) reports Lady Crabs as common on Lawrence (Ganong 1890). The Lady Crab is of consid- Cape Cod Bay sand flats (which commonly reach 20°C erable ecological importance as a consumer of mac- in summer). -

Narraguagus River Water Quality Monitoring Plan
Narraguagus River Water Quality Monitoring Plan A Guide for Coordinated Water Quality Monitoring Efforts in an Atlantic Salmon Watershed in Maine By Barbara S. Arter BSA Environmental Consulting And Barbara Snapp, Ph. D. January 2006 Sponsored By The Narraguagus River Watershed Council Funded By The National Fish and Wildlife Foundation Narraguagus River Water Quality Monitoring Plan A Guide for Coordinated Water Quality Monitoring Efforts in an Atlantic Salmon Watershed in Maine By Barbara S. Arter BSA Environmental Consulting And Barbara Snapp, Ph. D. January 2006 Sponsored By The Narraguagus River Watershed Council Funded By The National Fish and Wildlife Foundation Narraguagus River Water Quality Monitoring Plan Preface In an effort to enhance water quality monitoring (WQM) coordination among agencies and conservation organizations, the Project SHARE Research and Management Committee initiated a program whereby river-specific WQM Plans are developed for Maine rivers that currently contain Atlantic salmon populations listed in the Endangered Species Act. The Sheepscot River WQM Plan was the first plan to be developed under this initiative. It was developed between May 2003 and June 2004. The Action Items were finalized and the document signed in March 2005 (Arter, 2005). The Narraguagus River WQM Plan is the second such plan and was produced by a workgroup comprised of representatives from both state and federal government agencies and several conservation organizations (see Acknowledgments). The purpose of this plan is to characterize current WQM activities, describe current water quality trends, identify the role of each monitoring agency, and make recommendations for future monitoring. The project was funded by the National Fish and Wildlife Foundation. -

A History of Oysters in Maine (1600S-1970S) Randy Lackovic University of Maine, [email protected]
The University of Maine DigitalCommons@UMaine Darling Marine Center Historical Documents Darling Marine Center Historical Collections 3-2019 A History of Oysters in Maine (1600s-1970s) Randy Lackovic University of Maine, [email protected] Follow this and additional works at: https://digitalcommons.library.umaine.edu/dmc_documents Part of the Aquaculture and Fisheries Commons, History of Science, Technology, and Medicine Commons, and the United States History Commons Repository Citation Lackovic, Randy, "A History of Oysters in Maine (1600s-1970s)" (2019). Darling Marine Center Historical Documents. 22. https://digitalcommons.library.umaine.edu/dmc_documents/22 This Newsletter is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Darling Marine Center Historical Documents by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. A History of Oysters in Maine (1600s-1970s) This is a history of oyster abundance in Maine, and the subsequent decline of oyster abundance. It is a history of oystering, oyster fisheries, and oyster commerce in Maine. It is a history of the transplanting of oysters to Maine, and experiments with oysters in Maine, and of oyster culture in Maine. This history takes place from the 1600s to the 1970s. 17th Century {}{}{}{} In early days, oysters were to be found in lavish abundance along all the Atlantic coast, though Ingersoll says it was at least a small number of oysters on the Gulf of Maine coast.86, 87 Champlain wrote that in 1604, "All the harbors, bays, and coasts from Chouacoet (Saco) are filled with every variety of fish. -

2021 Striped Bass Regulations
2021 MAINE STRIPED BASS REGULATIONS If you are a recreational saltwater fisherman, Maine law may require you to register with the Maine Saltwater Recreational Fishing Registry. To learn more or to register visit: www.maine.gov/saltwater or call 207-633-9500. The following Maine saltwater recreational fishing regulations are current as of June 8, 2021. However, they are subject to change. Please contact our office or your local Marine Patrol Officer with questions. All minimum lengths are total length, NOT fork length. The sale of fish by recreational anglers is prohibited. Maine’s striped bass regulations cover all Maine coastal waters up to the head of tide in all rivers. In addition, there are special regulations in effect from December 1 through June 30 in the Kennebec, Sheepscot and Androscoggin Rivers and all related tributaries (see “SPECIAL KENNEBEC REGULATIONS” below). FEDERAL REGULATION It is unlawful to fish for, take or possess striped bass in Federal waters (waters greater than 3 miles from shore). STATEWIDE REGULATIONS OPEN SEASON January 1 through December 31, inclusive (except the Kennebec watershed, see below). BAG LIMITS A person may take and possess 1 fish per day. SIZE LIMITS The fish must be equal to or greater than 28 inches and less than 35 inches total length. “TOTAL LENGTH” is a straight line measurement from the lower jaw to the tip of the tail with the tail pinched together. DISPOSITION Personal use only, sale is prohibited. Fish must remain whole and intact. GENERAL GEAR RESTRICTIONS • Hook and line only, no gaffing of striped bass. • No bait allowed when using treble hooks. -

Dynamics of Larval Fish Abundance in Penobscot Bay, Maine
81 Abstract–Biweekly ichthyoplankton Dynamics of larval fi sh abundance surveys were conducted in Penobscot Bay, Maine, during the spring and early in Penobscot Bay, Maine summer of 1997 and 1998. Larvae from demersal eggs dominated the catch from late winter through spring, but Mark A. Lazzari not in early summer collections. Larval Maine Department of Marine Resources fi sh assemblages varied with tempera- P.O. Box 8 ture, and to a lesser extent, plankton West Boothbay Harbor, Maine 04575 volume, and salinity, among months. E-mail address: [email protected] Temporal patterns of larval fi sh abun- dance corresponded with seasonality of reproduction. Larvae of taxa that spawn from late winter through early spring, such as sculpins (Myoxocepha- lus spp.), sand lance (Ammodytes sp.), and rock gunnel (Pholis gunnellus) For most fi sh, the greatest mortality tial variation in species diver sity and were dominant in Penobscot Bay in occurs during early life stages (Hjort, abundance, and 3) to relate these vari- March and April. Larvae of spring to early summer spawners such as 1914; Cushing, 1975; Leggett and Deb- ations to differences in location and en- winter fl ounder (Pleuronectes america- lois, 1994). Therefore, it is essential that vironmental variables. nus) Atlantic seasnail (Liparis atlan- fi sh eggs and larvae develop in favorable ticus), and radiated shanny (Ulvaria habitats that maximize the probability subbifurcata) were more abundant in of survival. Bigelow (1926) recognized Materials and methods May and June. Penobscot Bay appears the signifi cance of the coastal shelf for to be a nursery for many fi shes; there- the production of fi sh larvae within the Field methods fore any degradation of water quality Gulf of Maine, noting that most larvae during the vernal period would have were found within the 200-m contour. -

Wright Pierce
SUBMISSION 05-03-16 CONFIDENTIAL KENNEBUNK LIGHT AND POWER DISTRICT HYDROPOWER FACILITY ALTERNATIVES ASSESSMENT STUDY MA9 2016 SUBMISSION 05-03-16 KENNEBUNK LIGHT AND POWER DISTRICT HYDROPOWER FACILITY ALTERNATIVES ASSESSMENT STUDY KENNEBUNK, MAINE TABLE OF CONTENTS SECTION DESCRIPTION PAGE 1 SUMMARY OF ALTERNATIVES 1.1 Prequel ..................................................................................... 1-1 1.2 Summary of Alternatives .......................................................... 1-3 1.3 Alternative #1 – Seek New License to Continue Operations ................................................................. 1-3 1.4 Alternative #2 – Seek License Exemption to Continue Operation ................................................................... 1-4 1.5 Alternative #3 – Seek FERC Non-jurisdiction Only for the Kesslen Site ................................................................... 1-5 1.6 Alternative #4 – Cease Operation and Surrender the FERC License for All Three Sites ............................................ 1-5 2 ASSESSMENT OF ALTERNATIVE #1 2.1 Alternative Description ............................................................ 2-1 2.2 Environmental Implications of this Alternative ....................... 2-2 2.3 Secondary Economic Implications of this Alternative ............ 2-7 2.4 Benefits of the Alternative ....................................................... 2-8 2.5 Impacts and Challenges of the Alternative .............................. 2-9 2.6 Cost Assessment of the Alternative ......................................... -

Shells of Maine: a Catalogue of the Land, Fresh-Water and Marine Mollusca of Maine
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Maine The University of Maine DigitalCommons@UMaine Maine History Documents Special Collections 1908 Shells of Maine: a Catalogue of the Land, Fresh-water and Marine Mollusca of Maine Norman Wallace Lermond Follow this and additional works at: https://digitalcommons.library.umaine.edu/mainehistory Part of the History Commons This Monograph is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Maine History Documents by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. Pamp 353 c. 2 Vickery SHELLS OF MAINE Norman Wallace Lermond Thomaston SHELLS OF MAINE. A Catalogue of the Land, Fresh-water and Marine Mollusca of Maine, by Norman Wallace Lermond. INTRODUCTORY. No general list of Maine shells—including land, fresh-water and marine species—-has been published since 1843, when Dr. J. W. Mighels’ list was printed in the Boston Journal of Natural History. Dr. Mighels may be called the “Pioneer” conchologist of Maine. By profession a physician, in his leisure hours he was a most enthusiastic collector and student of all forms of molluscan life. Enthusiasm such as his was “contagious” and he soon had gathered about him a little band of active students and collectors. Of these Capt. Walden of the U. S. Revenue Cutter “Morris” was dredging in deep water and exploring the eastern shores and among the islands, and “by his zeal procured many rare species;” Dr. -
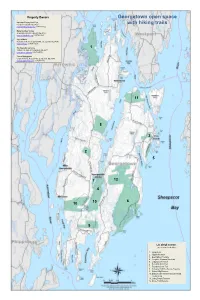
Georgetown Open Space with Hiking Trails
Property Owners Georgetown open space Kennebec Estuary Land Trust P.O. Box 1128, Bath, ME 04530 with hiking trails www.kennebecestuary.org (207-442-8400) Maine Audubon Society 20 Gisland Farm Rd., Falmouth, ME 04105 www.maineaudubon.org (207-781-2330) State of Maine Reid State Park, 375 Seguinland Rd., Georgetown, ME 04548 www.maine.gov (207-371-2303) The Nature Conservancy 1 14 Maine St., Suite 401, Brunswick, ME 04011 www.nature.org/maine (888-729-5181) Town of Georgetown 50 Bay Point Rd., P.O. Box 436, Georgetown, ME 04548 www.georgetownme.com (207-371-2820) 11 8 3 2 5 7 12 4 10 6 10 9 List of trail reserves (see reverse for details) 1. Flying Point 2. Higgins Mountain 3. Ipcar Natural Preserve 4. Josephine Newman Sanctuary 5. Ledgewood Preserve 6. Reid State Park Trail 7. Round the Cove Trail 8. Schoener Robinhood Cove Preserve 9. Weber Kelly Preserve 10. Berry Woods Preserve and James and Lavina Kemp 11. Loring Conant Preserve 12. Morse Pond Preserve Trail Descriptions 4 JOSEPHINE NEWMAN SANCTUARY 7 ROUND THE COVE TRAIL Trail Length: 2.6 miles, 3 trails, easy to moderate Trail Length: 1.4 miles, easy to moderate Owner: Town of Georgetown FLYING POINT Owner: Maine Audubon Society 1 Directions: From Route 127 in Georgetown Center turn right at the Directions: From Route 127 take Bay Point Road. Park at the Trail Length: 0.8 miles, moderate with hills sign for the sanctuary, shortly after crossing the first bridge on Georgetown Historical Society building on the left. The trail begins Owner: The Nature Conservancy Robinhood Cove. -

Lighthouses on the Coast of Maine Sixty-Seven Lighthouses Still Perch High on the Rocky Cliffs of Maine
™ Published since 1989 Where, when, and how to discover the best nature 116 photography in America Number 116 - October 2010 Cape Neddick Light - 62 mm / 93 All captions are followed by the lens focal length used for each photograph - DX and FX full-frame cameras. Lighthouses on the Coast of Maine Sixty-seven lighthouses still perch high on the rocky cliffs of Maine. Some of these lighthouses were built more than two hundred years ago to help sailors navigate their way through storms, fog, and dark of night. These beacons saved wooden merchant vessels sailing dangerous courses through narrow and shallow channels filled with countless hazards. Maine’s lighthouses were a part of our country’s history at a time when we were defending our shores, as far back as the Revolutionary war. Some were damaged by war and many were destroyed by the violence of nature. Light keepers risked their own lives to keep their lamps burning. A proud and dramatic beauty can be seen in these structures and their rugged environments–the reason I recently returned to Maine for another photo exploration. Issue 116 - page 2 You can fly into local airports like Portland or Whaleback Light Bangor, but fares are better and flights are more 43˚ 03’ 30” N frequent into Boston. You may want to rent a car 70˚ 41’ 48” W with a satellite navigation system or bring your From U.S. Route 1, drive east on State Route own portable GPS receiver. Just set your GPS 103 for 3.8 miles. Turn right onto Chauncey coordinates for the degrees/minutes/seconds Creek Road until you reach Pocahontas Road. -

Maine Revised Statutes 38 §467
Presented below are water quality standards that are in effect for Clean Water Act purposes. EPA is posting these standards as a convenience to users and has made a reasonable effort to assure their accuracy. Additionally, EPA has made a reasonable effort to identify parts of the standards that are not approved, disapproved, or are otherwise not in effect for Clean Water Act purposes. Maine Revised Statutes 38 §467 Maine Revised Statutes Title 38: WATERS AND NAVIGATION Chapter 3: PROTECTION AND IMPROVEMENT OF WATERS Subchapter 1: ENVIRONMENTAL PROTECTION BOARD Article 4-A: WATER CLASSIFICATION PROGRAM §467. Classification of major river basins All surface waters lying within the boundaries of the State that are in river basins having a drainage area greater than 100 square miles that are not classified as lakes or ponds are classified in this section. [1989, c. 764, §2 (AMD).] 1. Androscoggin River Basin. A. Androscoggin River, main stem, including all impoundments. (1) From the Maine-New Hampshire boundary to its confluence with the Ellis River - Class B. (2) From its confluence with the Ellis River to a line formed by the extension of the Bath-Brunswick boundary across Merrymeeting Bay in a northwesterly direction - Class C. [1989, c. 890, Pt. A, §40 (AFF); 1989, c. 890, Pt. B, §68 (AMD); MRSA T. 38, §467, sub-§1, ¶ A (AMD).] B. Little Androscoggin River Drainage. (1) Little Androscoggin River, main stem. (a) From the outlet of Bryant Pond to the Maine Central Railroad bridge in South Paris - Class A. (b) From the Maine Central Railroad bridge in South Paris to its confluence with the Androscoggin River - Class C. -

Atlantic Salmon EFH the Proposed EFH Designation for Atlantic Salmon
Atlantic salmon EFH The proposed EFH designation for Atlantic salmon includes the rivers, estuaries, and bays that are listed in Table 31 and shown in Map 105, which exhibit the environmental conditions defined in the text descriptions. Smaller tributaries not shown on the map are also EFH for one or more life stage as long as they conform to the proposed habitat descriptions. All EFH river systems form a direct connection to the sea, but EFH would not include portions of rivers above naturally occurring barriers to upstream migration or land-locked lakes and ponds. The oceanic component of EFH is to a distance of three miles from the mouth of each river. The new designation includes six new drainage systems not included in the original list of 26 rivers that were designated in 1998. All of them are in the Maine coastal sub-region (Chandler, Indian, Pleasant, St. George, Medomak, and Pemaquid rivers). All told, 30 river systems in nine New England sub-regions are designated for Atlantic salmon EFH. The new map includes a more continuous series of bays and areas adjacent to river mouths that are within three miles of the coast. Designated EFH in Long Island Sound has been reduced to small areas where the Connecticut and Pawcatuck Rivers empty into the sound, rather than taking up the entire sound. Also, there are a number of improvements in the text descriptions which make the habitat requirements for each life stage more specific and applicable to three separate juvenile life stages (fry, parr, and smolts). Text descriptions: Essential fish habitat for Atlantic salmon (Salmo salar) is designated as the rivers, estuaries, and bays that are listed in Table 31 and shown in Map 105. -

Morphodynamics of Tidal Inlet Systems in Maine
Maine Geological Survey Studies in Maine Geology: Volume 5 1989 Morphodynamics of Tidal Inlet Systems in Maine 1 2 Duncan M. FitzGeraui, Jonathan M. Lincoln • 3 1 L. Kenneth Fink, Jr. , and Dabney W. Caldwel/ 1Departm ent of Geology Boston University Boston, Massachusetts 02215 2Department of Geological Sciencies Northwestern University Evanston, Illinois 60201 3Department of Geology University of Maine Orono, Maine 04469 ABSTRACT The occurrence of tidal inlets along the coast of Maine is tied closely to the structural geology and glacial history of this region. Most of the inlets are found along the southern arcuate-embayment shoreline where sand sources, consisting of glaciomarine sediments and other glacial deposits, were sufficient to build swash-aligned barriers between pronounced bedrock headlands. Along the peninsula coast of Maine, tidal inlets also occur at the mouths of the Kennebec and Sheepscot Rivers where large quantities of glaciofluvial sands were deposited during deglacia tion. The remainder of the southeastward facing coast was stripped of its preglacial sediment cover by the southerly moving glaciers. The thin tills that were left behind yield little sand and, thus, barriers and inlets are generally absent. Small to large-sized inlets (width= 50-200 m) in Maine are anchored next to bedrock outcrops and are bordered on their opposite sides by sandy spits. Despite the ubiquitous name "river inlet," they normally have little fresh water discharge compared to their salt water tidal prisms. The backbarriers of these inlets are expansive and would produce relatively large tidal prisms if high Spartina marshes had not filled most of the region, leaving little open water area.