Probiotics in Chronic Inflammatory Bowel Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Between Umma and Dhimma the Christians of the Middle East Under the Umayyads
MINISTÈRE DE L'ÉDUCATION NATIONALE, DE L'ENSEIGNEMENT SUPÉRIEUR ET DE LA RECHERCHE ANNALES ISLAMOLOGIQUES en ligne en ligne en ligne en ligne en ligne en ligne en ligne en ligne en ligne en ligne AnIsl 42 (2008), p. 127-156 Arietta Papaconstantinou Between umma and dhimma. The Christians of the Middle East under the Umayyads. Conditions d’utilisation L’utilisation du contenu de ce site est limitée à un usage personnel et non commercial. Toute autre utilisation du site et de son contenu est soumise à une autorisation préalable de l’éditeur (contact AT ifao.egnet.net). Le copyright est conservé par l’éditeur (Ifao). Conditions of Use You may use content in this website only for your personal, noncommercial use. Any further use of this website and its content is forbidden, unless you have obtained prior permission from the publisher (contact AT ifao.egnet.net). The copyright is retained by the publisher (Ifao). Dernières publications 9782724708288 BIFAO 121 9782724708424 Bulletin archéologique des Écoles françaises à l'étranger (BAEFE) 9782724707878 Questionner le sphinx Philippe Collombert (éd.), Laurent Coulon (éd.), Ivan Guermeur (éd.), Christophe Thiers (éd.) 9782724708295 Bulletin de liaison de la céramique égyptienne 30 Sylvie Marchand (éd.) 9782724708356 Dendara. La Porte d'Horus Sylvie Cauville 9782724707953 Dendara. La Porte d’Horus Sylvie Cauville 9782724708394 Dendara. La Porte d'Hathor Sylvie Cauville 9782724708011 MIDEO 36 Emmanuel Pisani (éd.), Dennis Halft (éd.) © Institut français d’archéologie orientale - Le Caire Powered by TCPDF (www.tcpdf.org) 1 / 1 AriettA PapaconstAntinou Between Umma and Dhimma The Christians of the Middle East under the Umayyads “ ross and crescent”, “Church and Mosque”, “Bible and Qur’ān”: students of the Christians and Jews living in Islamic lands have consistently used religious metaphors Cto describe their object of study1—and indeed, this object has been overwhelmingly religious and, more specifically, theological in the broader sense. -

Santa Maria Antiqua: the Amalgamation of Identity in Early Medieval Rome
Pursuit - The Journal of Undergraduate Research at The University of Tennessee Volume 6 Issue 1 Article 7 April 2015 Santa Maria Antiqua: The Amalgamation of Identity in Early Medieval Rome Cayce Davis University of Tennessee, Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/pursuit Part of the Architectural History and Criticism Commons, and the Historic Preservation and Conservation Commons Recommended Citation Davis, Cayce (2015) "Santa Maria Antiqua: The Amalgamation of Identity in Early Medieval Rome," Pursuit - The Journal of Undergraduate Research at The University of Tennessee: Vol. 6 : Iss. 1 , Article 7. Available at: https://trace.tennessee.edu/pursuit/vol6/iss1/7 This Article is brought to you for free and open access by Volunteer, Open Access, Library Journals (VOL Journals), published in partnership with The University of Tennessee (UT) University Libraries. This article has been accepted for inclusion in Pursuit - The Journal of Undergraduate Research at The University of Tennessee by an authorized editor. For more information, please visit https://trace.tennessee.edu/pursuit. Pursuit: The Journal of Undergraduate Research at the University of Tennessee Copyright © The University of Tennessee PURSUIT trace.tennessee.edu/pursuit Santa Maria Antiqua: The amalgamation of Identity in Early Medieval Rome CAYCE DAVIS Advisor: Dr. Gregor Kalas The intent of this investigation is to frame an identity for the church of Santa Maria Antiqua and the urban condition of Rome during the sixth through eighth centuries. Coupling topographical and semiotic information with larger geographic issues, this study interrogates the church and specific individuals associated with it as a way of more comprehensively understanding Santa Maria Antiqua as a visual medium of cultural change and political propaganda. -

Christian Apologetics and the Gradual Restriction of Dhimmi Social Religious Liberties from the Arab-Muslim Conquests to the Abbasid Era Michael J
Eastern Michigan University DigitalCommons@EMU Master's Theses, and Doctoral Dissertations, and Master's Theses and Doctoral Dissertations Graduate Capstone Projects 2017 Shifting landscapes: Christian apologetics and the gradual restriction of dhimmi social religious liberties from the Arab-Muslim conquests to the Abbasid era Michael J. Rozek Follow this and additional works at: http://commons.emich.edu/theses Part of the History Commons Recommended Citation Rozek, Michael J., "Shifting landscapes: Christian apologetics and the gradual restriction of dhimmi social religious liberties from the Arab-Muslim conquests to the Abbasid era" (2017). Master's Theses and Doctoral Dissertations. 863. http://commons.emich.edu/theses/863 This Open Access Thesis is brought to you for free and open access by the Master's Theses, and Doctoral Dissertations, and Graduate Capstone Projects at DigitalCommons@EMU. It has been accepted for inclusion in Master's Theses and Doctoral Dissertations by an authorized administrator of DigitalCommons@EMU. For more information, please contact [email protected]. Shifting Landscapes: Christian Apologetics and the Gradual Restriction of Dhimmī Social-Religious Liberties from the Arab-Muslim Conquests to the Abbasid Era by Michael J. Rozek Thesis Submitted to the Department of History Eastern Michigan University in fulfillment of the requirements for the degree of MASTER of History in History Thesis Committee: Philip C. Schmitz, Ph.D, Chair John L. Knight, Ph.D April 21, 2017 Ypsilanti, Michigan Abstract This historical research study explores the changes of conquered Christians’ social-religious liberties from the first interactions between Christians and Arab-Muslims during the conquests c. A.D. 630 through the the ‘Abbasid era c. -
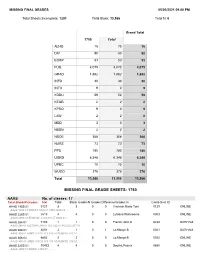
Missing Final Grade Sheets: 1750
MISSING FINAL GRADES 05/03/2021 09:00 PM Total Sheets Incomplete: 1251 Total Blank: 13,586 Total N: 0 Grand Total 1750 Total ALHG 15 15 15 DIV 90 90 90 EGRP 53 53 53 FUQ 4,075 4,075 4,075 GRAD 1,882 1,882 1,882 INTR 30 30 30 INTU 9 9 9 KDDU 59 59 59 KEGR 2 2 2 KPRO 9 9 9 LAW 2 2 2 MED 3 3 3 NBSN 2 2 2 NSOE 368 368 368 NURS 73 73 73 PPS 180 180 180 UGRD 6,348 6,348 6,348 UPBC 10 10 10 WUGD 376 376 376 Total 13,586 13,586 13,586 MISSING FINAL GRADE SHEETS: 1750 AAAS No. of classes: 17 Total Blank/N Grades: 104 Total Blank Grades N Grades Difference/Grades In Comb Sect ID AAAS 190S 01 3127 3 3 0 0 Cochran,Marie Toni 0129 ONLINE AAAS 190S.01 DOCST 190S.01 VMS 190S.02 . AAAS 228S 01 5519 4 4 0 0 Lubiano,Wahneema 0003 ONLINE AAAS 228S.01 ENGLISH 379S.01 LIT 382S.01 . AAAS 264 01 7109 1 1 0 0 French,John D 0234 DURHAM AAAS 264.01 HISTORY 264.01 ICS 232.01 POLSCI 257.01 . AAAS 306 01 6651 2 1 0 1 Lo,Mbaye B 0021 DURHAM AAAS 306.01 AMES 304.01 ICS 306.01 PUBPOL 305.01 . AAAS 306 02 6653 2 2 0 0 Lo,Mbaye B 0022 ONLINE AAAS 306.02 AMES 304.02 ICS 306.02 PUBPOL 305.02 . -

Surface Treatment Countycode Trafficgroup Route Lane Mpfromdesc Mptodesc Fromintersection Tointersection Nooflanes 34 VIII 624 B
Surface Treatment CountyCode TrafficGroup Route Lane MPFromDesc MPToDesc FromIntersection ToIntersection NoOfLanes 0.20 Miles: Belle Grove Rd; SC-727N/S 1.10 Miles: Veterans Rd; SC-625S/N 34 VIII 624 B 0.2 MS of Rt. 727 Rt. 625 (Frederick County); SC-727N/S (Frederick (Frederick County); SC-625S/N (Frederick 2 County) County) 34 V 625 B Rt. 624 0.065 M N of Rt. 635 2 34 VII 625 B Rte 11 Rte 624 2 34 VI 625 B Rte 627 Rte 631 2 2.40 Miles: N Frederick Pike; US-522N; US- 2.40 Miles: N Frederick Pike; US-522N; US- 34 VII 694 B End of Hard Surface Rte 522 2 522N 522N 34 V 695 B Rte 522 End of Hard Surface 0 Miles: N Frederick Pike; US-522S; US-522S 0 Miles: N Frederick Pike; US-522S; US-522S 2 3.89 Miles: N Frederick Pike; N Timber Ridge 34 VII 696 B Rte 522 WV Line 6.20 Miles: N Timber Ridge Rd; JB:W VA LINE 2 Rd; US-522N; Overlap Terminus:NORTH 0 Miles: Bloomery Pike; VA-127W/E; VA- 0.23 Miles: Omps Rd; SC-698N/S (Frederick 34 IV 696 B Rte 127 Rte 698 2 127W/E County) 0.13 Miles: S Timber Ridge Rd; SC-696N/S 0 Miles: Bloomery Pike; VA-127W/E; VA- 34 IV 698 B Rte 127 Rte 696 (Frederick County); SC-696N/S (Frederick 2 127W/E County) 0.70 Miles: Meadow Mills Rd; SC-624E/W 34 V 727 B Rt. -

Durham E-Theses
Durham E-Theses Nec silentio praetereundum: the signicance of the miraculous in the Anglo-Saxon church in the time of Bede Hustler, Jonathan Richard How to cite: Hustler, Jonathan Richard (1997) Nec silentio praetereundum: the signicance of the miraculous in the Anglo-Saxon church in the time of Bede, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/4991/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk 2 Nec silentio praetereundum: The significance of the Miraculous in the Anglo-Saxon Church in the Time of Bede by Jonathan Richard Hustler. Thesis submitted for the degree of Doctor of Philosophy University of Durham Department of History 1997 The copyright of this thesis rests with the author. No quotation from it should be published without the written consent of the author and information derived from it should be acknowledged. -

1 the Study of Islam's Origins Since W. Montgomery Watt's Publications Fred M. Donner
The Study of Islam’s Origins since W. Montgomery Watt’s Publications Fred M. Donner (The University of Chicago) Presented Friday, November 23, 2015, at the University of Edinburgh I wish to thank Dr. Anthony Gorman of Islamic and Middle Eastern Studies, and Professor Hugh Goddard of the Prince Alwaleed bin Talal Centre, and their colleagues for honoring me with the invitation to speak at this important occasion, celebrating Professor William Montgomery Watt’s long and distinguished career at Edinburgh. Montgomery Watt (1909-2006) was one of the most important and respected scholar of Islamic studies alive when I was beginning my scholarly career in the late 1960s and early 1970s—certainly, he was one of the most important for me, although, unfortunately, I never had the opportunity to meet him in person. His numerous studies—above all his works on the prophet Muḥammad1 and his several short introductory volumes in the Edinburgh University Press’s “Islamic Surveys” series (which, I believe, he may have instigated), especially his Islamic Philosophy and Theology (1962) and Islamic Political Thought: the basic concepts (1968)—were, on the one hand, models of lucid, careful scholarship and, on the other, incredibly helpful introductions to various topics within Islamic studies. Without his work to learn from and absorb, I know that my own development as a scholar would have been far more difficult, and much less pleasant. 1 W. Montgomery Watt, Muhammad at Mecca (Oxford: Clarendon Press, 1953); idem, Muhammad at Medina (Oxford: Clarendon Press, 1956); idem, Muhammad, Prophet and Statesman (Oxford: Oxford University Press, 1961). -

2014-2015 and Is Accurate and Current, to the Greatest Extent Possible, As of June 2014
Cover Cover 1 University’s Mission Statement James B. Duke’s founding Indenture of Duke University directed the members of the University to “provide real leadership in the educational world” by choosing individuals of “outstanding character, ability and vision” to serve as its officers, trustees and faculty; by carefully selecting students of “character, determination and application;” and by pursuing those areas of teaching and scholarship that would “most help to develop our resources, increase our wisdom and promote human happiness.” To these ends, the mission of Duke University is to provide a superior liberal education to undergraduate students, attending not only to their intellectual growth but also to their development as adults committed to high ethical standards and full participation as leaders in their communities; to prepare future members of the learned professions for lives of skilled and ethical service by providing excellent graduate and professional education; to advance the frontiers of knowledge and contribute boldly to the international community of scholarship; to promote an intellectual environment built on a commitment to free and open inquiry; to help those who suffer, cure disease and promote health, through sophisticated medical research and thoughtful patient care; to provide wide ranging educational opportunities, on and beyond our campuses, for traditional students, active professionals and life-long learners using the power of information technologies; and to promote a deep appreciation for the range of human difference and potential, a sense of the obligations and rewards of citizenship, and a commitment to learning, freedom and truth. By pursuing these objectives with vision and integrity, Duke University seeks to engage the mind, elevate the spirit, and stimulate the best effort of all who are associated with the University; to contribute in diverse ways to the local community, the state, the nation and the world; and to attain and maintain a place of real leadership in all that we do. -

A Plate of Anglo-Saxon Coins Found at Reculver, Kent in the Eighteenth Century
126 SHORT ARTICLES AND NOTES are difficulties arising from their similar distribution- their designs are not as unambiguous as this precious pattern. coin. The implications could spread some way Against that numismatic background, how should through the sceatta series. Among the varieties we assess the 'benediction hand' coin historically? attributable to Canterbury, should we be looking for a The primary phase of English sceattas had established four-to-two, or even a four-to-three ratio of royal to a tradition of coins with a head or bust in sub-classical ecclesiastical coins? The only touchstone upon which style on the obverse.15 Although it was obviously not we could test the plausibility of such ratios is the a portrait, the assumption that this was an icon of the proportion of coins of Iaenberht to those of Offa king would follow easily in people's minds. In Series alone, and the corresponding proportions for Ecg- K, beginning soon after the end of the primary phase, berht and Eadberht. It is difficult to see the Kentish a distinction is made: the bust is accompanied by kings surrendering a third or more of the profits of either a hawk or, commonly, a cross. The hawk is minting, but perhaps such profits were only a modest plainly a secular symbol, apt for a king, whereas the proportion of their total income from various sources, cross is of universal relevance. There are far too many e.g. tolls. The hostility between Offa and Iasnberht sceattas with crosses for us to contemplate giving may have curtailed the archbishop's average share for them all to ecclesiastical issuers. -

Department of Music Graduate Student Handbook 2017-2018
Department of Music Graduate Student Handbook 2017-2018 1 Table of Contents Prefatory Note ....................................................................................................... 4 Doctor of Philosophy Degree Requirements: Composition .................................. 5 Doctor of Philosophy Degree Requirements: Ethnomusicology ........................... 8 Doctor of Philosophy Degree Requirements: Musicology .................................. 11 Regulations Governing Students Entering with Prior Graduate Work at Other Institutions ........................................................................................................... 16 Registration Requirements .................................................................................. 17 Departmental Series, Ensembles, and the Instrument Collection ....................... 21 Graduate Studies Office ...................................................................................... 22 Departmental Facilities and Services .................................................................. 23 Departmental Graduate Student Representatives .............................................. 24 Funding ............................................................................................................... 25 Diagnostic Examination (Composition & Musicology) ......................................... 28 Diagnostic Examination (Ethnomusicology) ........................................................ 30 Foreign Language Requirements ....................................................................... -

Mgcsa Classified Ads
MGCSA CLASSIFIED ADS FOR SALE FOR SALE FOR SALE FOR SALE 15 hp Motor w/Berkeley Used • 1983 Toro Greensmaster 3 Pump (3-phased) TORO 474 1" BRASS Olathe Fairway triplex riding greensmower. 14 Used 2 years QUICK COUPLER VALVES Core Pulverizer h.p. Kohler. Very good Reel stock, $700 or Best Offer with standard cover. (2) 6" Clay Valves 8 blade reels, 4 bolt adjust. $900 l Contact: Requires l A" valve key. JOHN MONSON Contact: Dan or Greg • 1986 National 84" Triplex $15.00 each. Long Prairie, CC St. Cloud Country Club mower, 16 h.p. Kohler engine, ex- Contact: cellent condition $800 DAVID WOOD Oxbow CC (320) 732-3696 • 1983 Columbia Utility Cart (evenings) (320) 253-1331 with box $300 (701) 588-4266 • 1987 Columbia Golf Cart. $400 • 1988 Horvick 20" Trailer FOR SALE Sprayer with 70 gal. tank with FOR SALE TfeeJet 744 electric controls. $800 FOR SALE • 40 Toro VT3 Cushman Trucksters 1991 Controllers $400 This equipment is for sale from Toro Fairway Aerator 1966 Runabout • 1 LTC Controller . .$600 the Fargo Park District. All $ - Model 9500 Great condition... 1,000 equipment is sold as is with no All controllers include steel Used one season. warranty. All equipment can be 1988 Dump 22HP w/Rops pedestal and control panel. Price is negotiable. Contact: viewed at Edgewood Golf Course $3,500 TOM FISCHER Contact: KEVIN CLUNIS Maintenance Shop between the Contact: RICK LaPORTE Edinburgh USA St. Croix National Golf Club hours of 7:00 a.m. and 3:30 p.m. Phone 235-8234 for appoint- Tartan Park (612) 424-8756 (715) 247-4260 ment. -

The Graphic Icons of Anastasios of Sinai Calum Samuelson Olivet Nazarene University, [email protected]
Olivet Nazarene University Digital Commons @ Olivet M.A. in Christian Thought Theology 5-2015 The Graphic Icons of Anastasios of Sinai Calum Samuelson Olivet Nazarene University, [email protected] Follow this and additional works at: https://digitalcommons.olivet.edu/theo_mact Recommended Citation Samuelson, Calum, "The Graphic Icons of Anastasios of Sinai" (2015). M.A. in Christian Thought. 1. https://digitalcommons.olivet.edu/theo_mact/1 This Thesis is brought to you for free and open access by the Theology at Digital Commons @ Olivet. It has been accepted for inclusion in M.A. in Christian Thought by an authorized administrator of Digital Commons @ Olivet. For more information, please contact [email protected]. THE GRAPHIC ICONS OF ANASTASIOS OF SINAI BY CALUM SAMUELSON B.A., Olivet Nazarene University, 2013 THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Arts in Christian Thought in the School of Graduate and Continuing Studies Olivet Nazarene University, 2015 Bourbonnais, Illinois Contents Acknowledgements ............................................................................................................ iv Abbreviations .......................................................................................................................v Introduction ..........................................................................................................................1 Chapter I: Theological, Artistic, and Political Development (325 – 685) .....................4 Section