Seqirus Template ABPI
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AFC WIMBLEDON Advertising and Sponsorship Opportunities 2020/21
AFC WIMBLEDON Advertising and Sponsorship Opportunities 2020/21 AFC WIMBLEDON Advertising and Sponsorship Opportunities 2020/21 AFC WIMBLEDON Advertising and Sponsorship Opportunities 2020/21 BE PART OF OUR MIRACLE It’s one of the beautiful game’s greatest stories, an Now, the next part of our miracle is here: we are impossible dream come true. This is your unique going home. It’s been 29 years since we last played opportunity to become part of our real football at our famous old ground - Plough Lane. Against all miracle. the odds, we are due to move into a new stadium, just a stone’s throw from the old ground, in October, Eighteen years ago, AFC Wimbledon rose from the 2020. ashes of Wimbledon FC. We came out of adversity and through grit, determination and an enduring This completes the circle. passion for our club, we are now globally recognised This completes Wimbledon. for proving what the power of fans can achieve. Many of our commercial partners have been with us We’ve taken our club from the ninth tier of English for most, if not all, of our incredible journey. Our shirt football to the Football League in just nine years and sponsorship with ‘Football Manager’ is the longest- are now firmly established in League One - just two running in English football and is still going strong promotions from the Premier League. after 18 years. AFC WIMBLEDON Advertising and Sponsorship Opportunities 2020/21 With the new stadium close to completion, we now have many opportunities for new partners. Our ethos will remain the same. -

Wimbledon FC to Milton Keynes This Summer Is a Critical Moment in London’S Football History
Culture, Sport and Tourism Away from home Scrutiny of London’s Football Stadiums June 2003 Culture, Sport and Tourism Away from home Scrutiny of London’s Football Stadiums June 2003 copyright Greater London Authority June 2003 Published by Greater London Authority City Hall The Queen’s Walk London SE1 2AA www.london.gov.uk enquiries 020 7983 4100 minicom 020 7983 4458 ISBN 1 85261 496 1 Cover photograph credit EMPICS Sports Photo Agency This publication is printed on recycled paper Chair’s Foreword The move by Wimbledon FC to Milton Keynes this summer is a critical moment in London’s football history. This move prompted the London Assembly’s Culture, Sport and Tourism committee to look into the issue of redevelopment for London clubs. With Fulham and Brentford yet to secure new stadiums for their clubs and question marks remaining over Arsenal’s and Tottenham’s grounds the issue is a live one. We do not want to see more clubs leave London. During the 2002/03 season about 5 million fans watched professional football in London. In addition, hundreds of thousands of Londoners participate every year in club sponsored community projects and play football. This report seeks to ensure that this added value isn’t lost to Londoners. We did not set out to judge local situations but to tease out lessons learnt by London football clubs. Football is more than just a business: the ties that a club has with its area and the fans that live or come from there are great. We recommend that more clubs have supporters on their board and applaud the work of Supporters Direct in rejuvenating the links between clubs and their fan base. -

For Wimbledon's Football Club
a proposed new community stadium for Wimbledon’s football club “Returning to a stadium in Plough Lane would not only be the culmination of an astonishing rebirth for Wimbledon’s football club, it would also create a significant community asset for Merton.” “This brochure outlines our proposal Kingsmeadow, in Kingston upon “As a community-owned club we are to create a new community stadium Thames. We need a new stadium run in a prudent and sustainable way. in Plough Lane, Merton. As part of to allow more people to watch our We have demonstrated that football the local council’s “call for sites” matches, to improve the matchday clubs can be a powerful force for process, we are asking for the site, experience for our supporters, to good in their communities. Re-locating currently the location of a run-down extend our potential revenue through finally back to the area we are proud to greyhound stadium, to be designated sponsorship and corporate initiatives represent will enable us to extend the for use as a football ground. and to provided much-needed facilities range of activities we run, support and for hosting private events. encourage. “A new stadium development will see League football return to Merton, it will “But AFC Wimbledon is not a normal “If you would like more information allow AFC Wimbledon to grow and football club. We were founded by about our proposals, please look rise still further up the divisions and our fans and we are owned by our at the full documentation on the it will enable the club and the council fans. -

Stadia Commercial Brochure
STADIA THREE WIMBLEDON LONDON SW17 RETAIL & LEISURE TO LET LEISURE RETAIL 1,574 SQ.M. (16,939 SQ. FT. GIA) 1,018 SQ.M. (10,953 SQ. FT. GIA) WIMBLEDON GROUNDS, PLOUGH LANE, WIMBLEDON, LONDON SW17 0BL LEISURE RETAIL STADIA THREE PLOUGH LANE A 51,000 SQ.M. MIXED USE REGENERATION MASTERPLAN WITH NEW AFC WIMBLEDON STADIUM AND OVER 600 APARTMENTS STADIA THREE DEVELOPMENT OVERVIEW A major new landmark for Retail and split level Location, locality and South West London leisure space transport Stadia Three will be a regeneration showcase The entire ground level is to be occupied by retail and The development benefits from fast and direct road providing a new stadium for AFC Wimbledon leisure space, both with independent entrances, service and rail connections into central London while together with extensive residential, retail, areas and facilities. being situated deceptively close to the vast green recreational and cultural facilities. The The squash/leisure club could provide 5 courts, 2 expanses of Wimbledon Park, Wimbledon Common development will also accommodate a squash, gymnasiums and a dance studio together with and the largest of London’s royal parks - Richmond leisure or fitness club at ground and mezzanine comprehensive changing and locker facilities, ideally suited Park. The town centre lies little over one mile to level. for either an independent operator or brand chain. the south while Stadia Three will have four tube and rail lines within a 1300 metre radius. • Over 600 apartments above and • Retail unit providing 1018 sq.m. (10,953 sq.ft.) immediately adjacent prime retail GIA finished to shell. -

Wimbledon Greyhound Stadium Only
Site Proposal 37 Wimbledon Greyhound Stadium Plough Lane, Tooting, SW17 0BL and 46-76 Summerstown, London SW17 0BH Site area 5.29 ha Site description Approximately two thirds of the site is dominated by the Wimbledon Greyhound Stadium, the remainder of the site is a car park with some commercial and industrial uses. The buildings on the eastern boundary are in separate ownership and contain a light industrial use (Volante) and Elite motorcycle training fronting Summerstown. The building in the southeast corner contains a food establishment. The site also accommodates Christopher’s Squash and Fitness Club within the stadium buildings and a hand car wash accessed from adjacent Copper Mill Lane. Weekly car boot sales are also operated from the car park. The site adjoins an industrial estate along the northern and eastern boundary. To the south of the site in Merton on the other side of Plough Lane is an industrial estate. Running along the western boundary of the site is a large operational electricity substation owned by National Grid. Strategic planning factors The site and its surrounds are within the functional floodplain of the River Wandle (Flood Zone 3b). The majority of the site is within a critical drainage area for surface water flooding. Sites and Policies Plan | 315 The site is surrounded on all sides by strategic industrial locations. To the north and east of the site is Summerstown Road strategic industrial location (London Borough of Wandsworth), which includes a waste management site to the northwest. To the south and west is part of Durnsford Road/Plough Lane strategic industrial location (London Borough of Merton). -

Stadia Three Brochure
STADIA THREE WIMBLEDON LONDON SW17 A dynamic regeneration showcase STADIA THREE Offering some of the most luxurious new apartments in South West London... with the world’s most prestigious championships on their doorstep. Computer generated image of Stadia Three viewed across Plough Lane. 2 3 STADIA THREE Computer generated image of entire regeneration masterplan. 4 5 Today, Wimbledon is a prized location offering a prestigious lifestyle - with Stadia Three having been designed to further elevate this status to that of sheer exclusivity. Residents at Stadia Three will have the best of both worlds within easy reach: • 4 rail lines serving the Capital. • A mainline commute of just 13 Wimbledon minutes to Waterloo. • Wimbledon All England Lawn Tennis Awaken the senses & Croquet Club (home of The Championships) will be less that 8 minutes’ drive time. • Wimbledon Park will be little over 20 minutes’ walk providing a plethora of recreational pursuits amid its 67 acres of parkland and serene waters. • The vast swathe of Wimbledon Common and the largest of London’s Royal Parks, Richmond Park, also lie deceptively close to Stadia Three. • Richmond Park is also the home of the Royal Ballet School. Wimbledon Village is situated on the eastern fringe of the common, a niche pocket overflowing with European style café culture, indie boutiques, bars and fashionable eateries that so define its cosmopolitan character. 6 7 WIMBLEDON RICHMOND ALL ENGLAND LAWN TENNIS WIMBLEDON WIMBLEDON COMMON PARK & CROQUET CLUB PARK PARK STN. Computer generated model of development masterplan. 8 9 Putney Bridge Barnes Wandsworth Bridge A 2 0 5 U CLAPHAM P P E R Within 15 minutes by car, residents at Stadia Three can cross the Thames at R I C H M JUNCTION O N D Wandsworth Bridge; by tube, stop for a quick shop on Fulham Broadway; R O A D or by rail, alight at Waterloo and hop on the Jubilee line - whichever way Putney and whatever the direction, it’s fast, direct and interconnecting. -

Transcript for Culture, Sport and Tourism Meeting on 1 April 2003
Item 4 Appendix D Transcript for Culture, Sport and Tourism meeting on 1 April 2003. Chair: Welcome to this meeting of the London Assembly Culture, Sport and Tourism Committee. I’m Meg Hillier, the Chair of the Committee, and I’m joined by my colleagues Len Duvall and Mike Tuffrey. Danny Myers is the Committee Scrutiny Manager and Saba Master is the Committee Administrator. This is our second enquiry into the future of football stadia in London. Chair: There are nine witnesses here this evening; three from London’s football clubs, three from supporters trusts and supporters, and four from residents’ groups. Thank you very much for coming. Charles Koppel, please briefly outline why you saw the only future for Wimbledon FC to move to Milton Keynes? Charles Koppel: We did a considerable amount of work over a long period of time to find alternate locations for the club. A lot of that was done in partnership with Merton council, looking at sites within Merton. We also contacted approximately 35 other boroughs in and around South London and we appointed a leading planning and property firm to undertake a research report on our behalf. After all that came back with no options we realised we needed to look elsewhere. The Milton Keynes opportunity came along and we felt it appropriate to pursue it. Chair: You spent 12 years without a ground. How far a field were you prepared to go out of London? Charles Koppel: I’ve only been at the club for three years so I can’t speak for the previous nine, but it was a big issue for the club. -

Wimbledon Greyhound Stadium, Plough Lane in the London Borough of Merton Planning Application No
planning report D&P/3130b/02 22 March 2016 Wimbledon Greyhound Stadium, Plough Lane in the London Borough of Merton planning application no. 14/P4361 Strategic planning application stage II referral Town & Country Planning Act 1990 (as amended); Greater London Authority Acts 1999 and 2007; Town & Country Planning (Mayor of London) Order 2008. The proposal The proposals comprise the demolition of the existing buildings and the erection of a 20,000 seat football stadium (initially 11,000 seat) with hospitality and coach parking, pedestrian streets, 1,273 sq.m. retail unit, 1,730 sq.m. squash and fitness club, 602 residential units with basement parking, refuse storage, 297 car parking spaces, cycle parking, and associated landscaping/open space and servicing. The applicant The applicant is Galliard Homes and the architect is Sheppard Robson. Strategic issues The proposed sporting intensification of the site, to provide a professional sports venue enabled by mixed-use redevelopment comprising residential, improved squash club and fitness facilities and small scale retail is supported in principle by strategic and local planning policy. Issues regarding flood risk, density, design, transport, affordable housing, children’s play space, urban design and sustainable development have been suitably addressed through the submission of further information and/or the use of planning conditions and section 106 obligations. The Council’s decision In this instance Merton Council has resolved to grant permission. Recommendation That Merton Council be advised that the Mayor is content for it to determine the case itself, subject to any action that the Secretary of State may take, and does not therefore wish to direct refusal or direct that he is to be the local planning authority. -
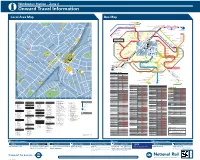
Local Area Map Bus Map
Wimbledon Station – Zone 3 i Onward Travel Information Local Area Map Bus Map 19 St. Mary’s Putney Bridge 25 93 18 40 1 C of E Church Wimbledon Park 16 7 D 17 S N 70 S E D A D D A 4 T Fellowship L A K E R O A D L R A O WALDEMAR ROAD O A . R River Thames G R 8 House E R O D O R A R O A D 1 N E M R R T O R U A St. Mary’s Church Putney Pier 219 4 S 26 E Clapham Junction Falcon Lane A A H C T P H Queenstown R T Nine Elms N 1 R KENILWORTH AVENUE A H Y A A Putney Exchange L O R 31 ’ Road C S T Lane 156 S 70 Queen Mary’s Roehampton L 1 PUTNEY R R 56 Putney CLAPHAM JUNCTION Westminster U O D University Hospital Medfield Street 60 14 A 59 Battersea Park 23 E H D U R Leopold RoadRoa 2 Clapham Junction RICARDS ROAD N O 36 G A P R O A D Lavender Hill Vauxhall 1 C E A ST. AUBYN’S AVENUE V 1 D 58 Bishop Gilpin A N87 L D O 85 Putney Heath Green Man Wandsworth Town Hall 85 22 15 P 1 Roehampton Primary School O 1 E 113 27 continues to 3 LANCASTER AVENUE L University Wandsworth LANCASTER ROAD 1 MARRYAT ROAD 106 Trafalgar Square 9 Wandsworth Southside Road A L A N R O A D Roehampton Lane Clapham Junction Northcote for Charing Cross 1 18 Tibbet’s Corner Rosslyn Park Rugby Club and Aldwych S Southfields Merton Road Southfields Academy N 1 D E O 1 D E L A D O R East Sheen A 26 10 H R D 1 38 O G 25 Durnsford Road Stroud Road LANCASTER ROAD U E D I Upper Richmond Road West Parkside Hospital S 2 K R Battersea Rise 12 E A A Wimbledon Park Road L N C S R U BELVEDERE AVENUE R S H L E M O B D Southdean Gardens E R D S HELME CLOSE Sheen Road Manor Road E E P 1 150 Wimbledon -

University of London Boat Club Boathouse, Chiswick
Played in London a directory of historic sporting assets in London compiled for English Heritage by Played in Britain 2014 Played in London a directory of historic sporting assets in London This document has been compiled from research carried out as part of the Played in London project, funded by English Heritage from 2010-14 Contacts: Played in Britain Malavan Media Ltd PO Box 50730 NW6 1YU 020 7794 5509 [email protected] www.playedinbritain.co.uk Project author: Simon Inglis Project manager: Jackie Spreckley English Heritage 1 Waterhouse Square, 138-142 Holborn, London EC1N 2ST 0207 973 3000 www.english-heritage.org.uk Project Assurance Officer: Tim Cromack If you require an alternative accessible version of this document (for instance in audio, Braille or large print) please contact English Heritage’s Customer Services Department: telephone: 0870 333 1181 fax: 01793 414926 textphone: 0800 015 0516 e-mail: [email protected] © Malavan Media Ltd. January 2015 malavan media Contents Introduction .................................................................................4 � 1 Barking and Dagenham.................................................................7 � 2 Barnet ........................................................................................8 � 3 Bexley ......................................................................................10 � 4 Brent ......................................................................................11 � 5 Bromley ....................................................................................13 -

Buses from Summerstown and Wimbledon
Buses from St. Georges Hospital Buses from Summerstown and Wimbledon Stadium 44 Battersea 77 G1 N155 155 towards Victoria Garratt Lane Rise towards Waterloo towards Battersea towards Aldwych towards Elephant & Castle Mapleton Road from stop HR from stop HR from stops HA, HC, HD, HE, from stops HN, HQ from stops HM, HN Garratt Lane HF, HG, HH, HR, HT, HV 270 Old Sergeant 219 Northcote Road Clapham Common 270 44 219 77 towardstowards Putney Putney Bridge Bridge Garratt Lane towards Victoria towards Waterlootowards Clapham Junction from stop HR Swafeld Road Wandsworth Common Spencer Park from stops HN, HQ from stops SA, SB, SC, SD, SE44 270 from stops SA, SB, SC, SD, SE from stops SA, SB, SC, SD, SE N44 North Side N44 77 CLAPHAM towards Aldwych Earlseld N44 Trinity Road G1 Broomwood Road 155 N155 RoadtowardsAlexander Aldwych Court towards Battersea Shaftesbury Estate from stop HR Garratt Lane Riverside QuarterHenry Prince Estate from stops SA, SB, SC, SD, SE from stops SA, SB, SU, Unlettered, H&R1 Hail & Ride Clapham Common 270 South Side Trinity Trinity Road Trinity Road Trinity Road section Garratt Lane Road WindmillClapham Road County Junction Arms Routh Road PUTNEY Earlseld Road Wandsworth West 77 G1 Town Side CLAPHAM Wandsworth Clapham South Earlseld Clapham Junction Northcote JUNCTION Common G1 Nightingale G1 Garratt Lane Springeld Isis Street Burntwood Trinity Road Lane Battersea Rise Lane University Burntwood Lane Garratt Lane NorthcoteHospital Road Nightingale Wandsworth Waldron RoadSpencer Park Broomwood Road G1 Trinity RoadLane Balham Hill Brodrick Road Southside Shopping Centre 44 270Garratt N44 Lane Hail & Ride Clapham 219 St. -

AFC Wimbledon
SPORTS PROJECT CASE STUDY AFC Wimbledon About AFC Wimbledon Wimbledon FC – not to be confused with AFC Wimbledon – played at the Plough Lane stadium for over 80 years. However, in 2001, a consortium of businessmen persuaded them to relocate the club to Milton Keynes – over 60 miles away. After the move they also changed their name to Milton Keynes Dons FC. A large number of fans weren’t happy with this move and decided to launch a new club in Wimbledon and start all over again at the bottom of English football. AFC Wimbledon was born. Even though they couldn’t play at Plough Lane any longer and had to share grounds with Kingstonian FC and QPR, the new club’s fans stuck with them. Their loyalty was quickly rewarded. AFC Wimbledon was promoted six times in thirteen seasons – taking them from the ninth tier to the third – League One. They’re also the first club formed in the 21st century to make it into the Football League too. On November 3rd, 2020, they returned to Wimbledon and hosted their first match at the newly built, Midstream lit, Plough Lane home. Find out more: midstreamlighting.com or call +44 (0) 207 584 8310 At a glance Sector: Football Project date: October 2020 Lux Average Values: 500 Lux Total power absorbed: 52.4kW Country: UK LED floodlights installed: 52 Uniformity: 0.7 Club founded: 2002 Customer: AFC Wimbledon Type: Modus R Series Nominal power for a single appliance: 1,200W Stadium capacity: 9,300 Our in-house lighting design team also future-proofed the whole solution.