? Repsi-Mob28 Riots Oritnic Pdow1169 Y 8627 Bp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Isolation and Morphological Characterization of Novel Bacterial
BIOLOGIA (PAKISTAN) PKISSN 0006 – 3096 (Print) June, 2018, 64 (1), 119-128 ISSN 2313 – 206X (On-Line) Isolation and morphological characterization of novel bacterial Endophytes from Citrus and evaluation for antifungal potential against Alternaria solani SEHRISH MUSHTAQ1, MUHAMMAD SHAFIQ1, MUHAMMAD ASHFAQ*1 FAIZA KHAN1, SOHAIB AFZAAL1, UMMAD HUSSAIN1, MUBASSHIR HUSSAIN1, RASHID MUKHTAR BALAL2 & MUHAMMAD SALEEM HAIDER1 1 Institute of Agricultural Sciences, University of the Punjab, Quaid-e-Azam Campus, Lahore, Pakistan. 2Department of Horticulture, University of Sargodha, Sargodha, Pakistan ABSTRACT Endophytes have always been a topic of interest for researchers due to their wide variety of benefits to their hosts and their diversity of geographical distribution. In this study, bacterial endophytes were isolated from the leaf midrib of different varieties of citrus cultivated in the Sargodha region of Punjab Pakistan. The endophytic bacterial community associated with citrus was characterized and screened for antifungal activity against Alternaria solani which causes losses to crops. A total of twelve strains were identified based on morphological and biochemical tests following Bergey’s manual of systematic bacteriology. The antagonistic potential of bacterial endophytes to A. solani was explored using the agar absorption method. This study showed the antifungal potential of Pantoea sp. (35.66%), Ensifer adhaerens (35.33%), Citrobacter diversus (33.03%), and Azotobacter nigricans (31.56%) to check the growth of pathogenic fungi compared to controls. Aureobacterium liquifaciens (27.66%), Acinetobacter sp. (25.66%), Bordetella pertussis (26.63%) also showed equal potential for inhibition. In contrast remaining isolates Enterobacter cloacae (19.33%), Azomonas agilis (17%) and Kurthia sp. (19%) were less efficient as compared to the others. -

Hoffman Dissertation.Pdf
Determining the spatial and seasonal influences of microbial community composition and structure from the Hawaiian anchialine ecosystem by Stephanie Kimie Hoffman A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama December 10, 2016 Keywords: anchialine, microbial ecology, Hawaii, laminated mat, spatial variation, seasonal variation Copyright 2016 by Stephanie Kimie Hoffman Approved by Scott R Santos, Chair, Professor of Biological Sciences Mark R Liles, Professor of Biological Sciences Todd D Steury, Associate Professor of Wildlife Sciences Robert S Boyd, Professor and Undergraduate Program Officer of Biological Sciences Abstract Characterized as coastal bodies of water lacking surface connections to the ocean but with subterranean connections to the ocean and groundwater, habitats belonging to the anchialine ecosystem occur worldwide in primarily tropical latitudes. Such habitats contain tidally fluctuating complex physical and chemical clines and great species richness and endemism. The Hawaiian Archipelago hosts the greatest concentration of anchialine habitats globally, and while the endemic atyid shrimp and keystone grazer Halocaridina rubra has been studied, little work has been conducted on the microbial communities forming the basis of this ecosystem’s food web. Thus, this dissertation seeks to fill the knowledge gap regarding the endemic microbial communities in the Hawaiian anchialine ecosystem, particularly regarding spatial and seasonal influences on community diversity, composition, and structure. Briefly, Chapter 1 introduces the anchialine ecosystem and specific aims of this dissertation. In Chapter 2, environmental factors driving diversity and spatial variation among Hawaiian anchialine microbial communities are explored. Specifically, each sampled habitat was influenced by a unique combination of environmental factors that correlated with correspondingly unique microbial communities. -

Diversity of Diazotrophic Bacteria in Greenwater System of Coastal Aquaculture
Journal of ISSN: 2319-8745 Environmental and Applied Bioresearch (An International open access online journal) www.appliedbioresearch.com Original article Diversity of diazotrophic bacteria in greenwater system of coastal aquaculture Velusamy Kathiravana and Kishore Kumar Krishnanib* aCentral Institute of Brackishwater Aquaculture, 75, Santhome High Road, R.A. Puram, Chennai, 600028, India bSchool of Edaphic Stress Management, National Institute of Abiotic Stress Management, Baramati (Dist. Pune) 413115 India * Corresponding author: [email protected] Keywords: Abstract: Greenwater; Coastal aquaculture; nifH genes; As atmospheric nitrogen (N2) cannot be directly utilized, it must be reduced to ammonia (NH3) by the Diversity; Nitrogen fixing community of nitrogen-fixing bacteria. The gene encoding nifH is largely unique to nitrogen fixing bacteria. bacteria, as it converts atmospheric nitrogen into ammonia by producing dinitrogenase reductase enzyme. In the present study, water sample from greenwater system were analyzed using nifH gene as a Received on: 01.08.2012 biomarker. Metagenomic clone library was constructed for nifH genes. Clones exhibit 83-98% similarity Accepted on: 16.08.2012 Published on: at nucleotide level and 88-98% similarity at amino acid level with dominating cluster of gamma proteobacteria, other clusters of beta proteobacteria (Dechloromonas aromatica and Zoogloea oryzae), alpha proteobacteria (Bradyrhizobium japonicum), delta proteobacteria (Desulfovibrio sp.) and uncultured bacteria. Present study sheds light on the high level of species richness in the nitrogen fixing bacterial population in greenwater system of coastal aquaculture. 1. INTRODUCTION The finfish community belongs to euryhaline group of fishes that are grown in estuaries and creeks. These fishes have high In India, shrimp aquaculture is practiced extensively. -

Supplementary Information Deep Ocean Metagenomes Provides Insight Into the Metabolic Architecture of Bathypelagic Microbial Comm
Supplementary Information Deep ocean metagenomes provides insight into the metabolic architecture of bathypelagic microbial communities Authors: Silvia G. Acinas1§*, Pablo Sánchez§1, Guillem Salazar§1,2, Francisco M. Cornejo- Castillo§1,3, Marta Sebastián1,4, Ramiro Logares1, Marta Royo-Llonch1, Lucas Paoli2, Shinichi Sunagawa2, Pascal Hingamp5, Hiroyuki Ogata6, Gipsi Lima-Mendez7,8, Simon Roux9D, José M. González10, Jesús M. Arrieta11, Intikhab S. Alam12, Allan Kamau12, Chris Bowler13,14, Jeroen Raes15,16, Stéphane Pesant17,18, Peer Bork19, Susana Agustí20, Takashi Gojobori12, Dolors Vaqué1, Matthew B. Sullivan21, Carlos Pedrós-Alió22, Ramon Massana1, Carlos M. Duarte23, Josep M. Gasol1,24 FL Taxonomic group Archaea Bacteria Eukaryota undef Viruses 1.00 Relative counts Relative 0.75 0.50 0.25 0.00 PA 1.00 0.75 0.50 0.25 0.00 7 10 13 17 20 23 26 32 35 41 43 50 53 59 62 65 67 74 77 81 82 91 97 103 109 112 118 121 131 134 144 146 Station Supplementary Fig. 1. Relative taxonomic composition at the domain level (Archaea, Bacteria and Eukarya and Viruses) of the M-GeneDB genes found in each of the 58 bathypelagic metagenomes. FL, free-living size fraction (0.2-0.8 µm); PA, particle-attached size fraction (0.8-20 µm). KO count per KEGG metabolism in annotated MP-geneDB genes Count 30000 20000 10000 0 Function unknown Exosome [BR:ko04147] Transporters [BR:ko02000]Enzymes with EC numbers Secretion system [BR:ko02044] ABC transporters [PATH:ko02010] Quorum sensing [PATH:ko02024] Purine metabolism [PATH:ko00230] Ribosome biogenesis [BR:ko03009] -

Prokaryote Data We
Dataset S1. Endogenous respiration rates in heterotrophic prokaryotes Notes to Table S1a: On data collection: Prokaryote data were compiled by searching the www.pubmedcentral.nih.gov full-text library for "bacterium" and "endogenous respiration" and sub- sequent analysis of the returned 570 documents (mostly papers in the Journal of Bacteriology and Journal of Applied and Environmental Microbiol- ogy, time period 1940-2006) and references therein. On cell size and taxonomy: Data on endogenous respiration rates (i.e. respiration rates of non-growing cells in nutrient-deprived media) in heterotrophic eukaryotes are pre- sented. Studies of bacterial respiration very rarely report information on cell size, which had therefore to be retrieved from different sources. To do so, an attempt was made to assign the bacterial strains described in the metabolic sources to accepted species names, to futher estimate the cell size for these species in the relevant literature. This was done using strain designations and information in the offician bacterial culture collections, like ATCC (American Type Culture Collection), NCTC (National Type Culture Collection) and others. Column "Species (Strain)" gives the strain designation as given by the authors of the respiration data paper. Column "Valid Name" gives the relevant valid species name for this strain, as determined from culture collections' information and/or other literature sources. Valid names follow Euzéby (1997). Cell size in the "Mpg" column correspond to species indicated in the "Valid Name" column. Note that this information is of approxi- mate nature, because many respiration data come from quite old publications and it was sometimes difficult to find out the valid name of the strain used with great precision. -

Investigation Into the Microbiological Causes of Epizootics Of
Investigation into the microbiological causes of epizootics of Pacific oyster larvae (Crassostrea gigas) in commercial production by Christopher Chapman B. Ag. Sc (Hons) University of Tasmania Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy University of Tasmania Hobart Tasmania Australia February 2012 Declaration This thesis contains no material that has been accepted for the award of any other degree or diploma in any tertiary institution. To the best of my knowledge this thesis does not contain material written or published by another person, except where due reference is made. Christopher Chapman University of Tasmania Hobart February 2012 This thesis may be made available for loan and limited copying in accordance with the Copyright Act 1968 Acknowledgements To my supervisory team, Mark Tamplin, John Bowman, Shane Powell and Michel Bermudes I would like to thank you all. To Mark for his guidance and for keeping me true to my plan; to John (the walking Bergey’s manual) for his fresh ideas and for his encouragement; to Shane, with whom I worked closely on all aspects of this project, thanks especially for helping me survive the laboratory and for being critical on drafts of this thesis; and to Michel for providing the industry perspective. I would like to give particular thanks to the Shellfish Culture team to whom I promised more than I was able to deliver. They made me feel part of the team and gave me free run of their hatchery. Thanks to Tom Spykers and Lee Wilson whose time-tested observations informed the direction of this study. -

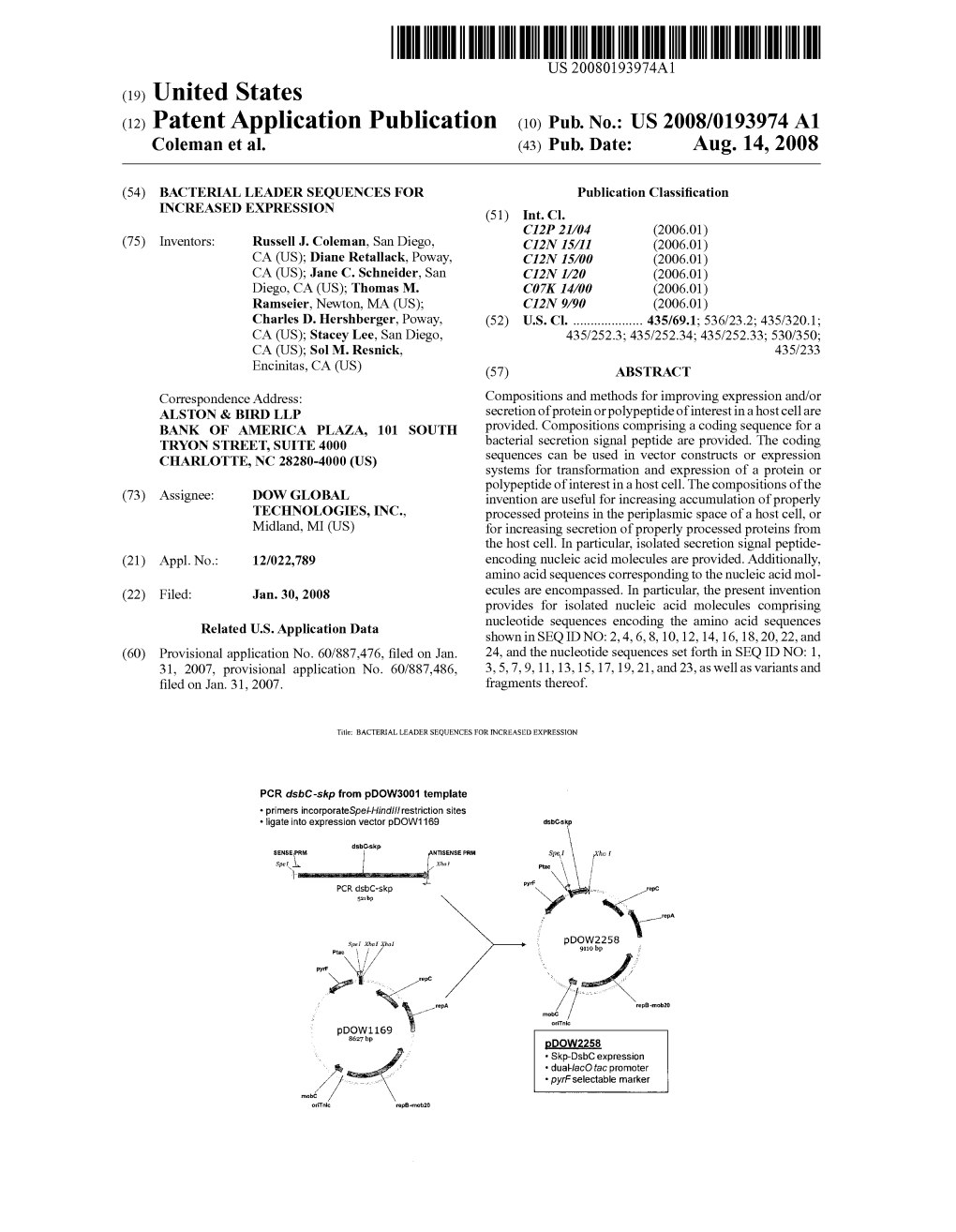
(12) Patent Application Publication (10) Pub. No.: US 2010/0048864 A1 Coleman Et Al
US 20100048864A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0048864 A1 Coleman et al. (43) Pub. Date: Feb. 25, 2010 (54) BACTERIAL LEADER SEQUENCES FOR Publication Classification INCREASED EXPRESSION (51) Int. Cl. (75) Inventors: Russell J. Coleman, San Diego, C07K I4/2 (2006.01) CA (US); Diane Retallack, Poway, CI2P 2L/06 (2006.01) CA (US); Charles D. Hershberger, CI2N I/2 (2006.01) Poway, CA (US); Stacey Lee, San CI2N 15/63 (2006.01) Diego, CA (US) C7H 2L/04 (2006.01) Correspondence Address: (52) U.S. Cl. ..................... 530/326; 435/69.1; 435/252.3: Alston & Bird LLP 435/252.33:435/320.1536/23.7 Dow Global Technologies, Inc. Bank Of America Plaza, 101 South Tryon Street, (57) ABSTRACT Suite 4000 Charlotte, NC 28280-4000 (US) Compositions and methods for improving expression and/or secretion of a polypeptide of interest in a host cell are pro (73) Assignee: Dow Global Technologies Inc., vided. Compositions including a coding sequence for a bac Midland, MI (US) terial Secretion signal peptide are provided. The compositions of the invention are useful for increasing accumulation of (21) Appl. No.: 12/604,061 properly processed proteins in the periplasmic space of a host cell, or for increasing secretion of properly processed pro (22) Filed: Oct. 22, 2009 teins. In particular, isolated secretion signal peptide-encoding nucleic acid molecules are provided. Additionally, amino Related U.S. Application Data acid sequences corresponding to the nucleic acid molecules are encompassed. The present invention provides for isolated (62) Division of application No. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0234346A1 Retallack Et Al
US 20060234346A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0234346A1 Retallack et al. (43) Pub. Date: Oct. 19, 2006 (54) EXPRESSION OF MAMMALIAN PROTEINS (60) Provisional application No. 60/564,798, filed on Apr. N PSEUDOMONAS FLUORESCENS 22, 2004. Provisional application No. 60/537,148, filed on Jan. 16, 2004. Provisional application No. 60/417,124, filed on Oct. 8, 2002. (76) Inventors: Diane M. Retallack, Poway, CA (US); Charles H. Squires, Poway, CA (US); Publication Classification David C. Watkins, East Greenwich, RI (51) Int. Cl. (US); Stacey L. Lee, San Diego, CA CI2P 2/06 (2006.01) (US); Frank H. Gaertner, San Diego, C07K I4/47 (2006.01) CA (US); Robert Shutter, Poway, CA CI2N I/2 (2006.01) (US) CI2N 15/74 (2006.01) C7H 2L/04 (2006.01) Correspondence Address: (52) U.S. Cl. ................... 435/69.1; 435/471; 435/252.34; KING & SPALDING LLP 530/350, 536/23.1 118O PEACHTREE STREET ATLANTA, GA 30309 (US) (57) ABSTRACT (21) Appl. No.: 11/400,840 The invention is a process for improved production of a recombinant mammalian protein by expression in a (22) Filed: Apr. 7, 2006 Pseudomonad, particularly in a Pseudomonas fluorescens organism. The process improves production of mammalian Related U.S. Application Data proteins, particularly human or human-derived proteins, over known expression systems such as E. coli in compa (63) Continuation of application No. 11/038,901, filed on rable circumstances Processes for improved production of Jan. 18, 2005, and which is a continuation-in-part of isolated mammalian, particularly human, proteins are pro application No. -

Physiological Investigations of Aerobic Petroleum Degradation in Marine Sediment Microcosms
Physiological investigations of aerobic petroleum degradation in marine sediment microcosms Florin Musat Physiological investigations of aerobic petroleum degradation in marine sediment microcosms Dissertation zur Erlangung des Grades eines Doktors der Naturwissenschaften − Dr. rer. nat. – dem Fachbereich Biologie/Chemie der Universität Bremen vorgelegt von Florin Musat aus Bukarest Bremen 2005 Die Untersuchungen zur vorliegenden Doktorarbeit wurden am Max-Planck-Institut für Marine Mikrobiologie in Bremen durchgeführt. 1. Gutachter: Prof. Dr. Friedrich Widdel, Universität Bremen 2. Gutachter: Prof. Dr. Heribert Cypionka, Universität Oldenburg Table of contents Abbreviations Summary 1 Part I. Biodegradation of crude oil and nitrogen fixation: an overview of the literature and results of the present study A.1. Oil pollution and biodegradation – a brief overview 3 1.1. Composition of crude oil 3 1.2. The fate of crude oil spilled into environment 6 1.3. Biodegradation of crude oil 7 1.3.1. Aerobic hydrocarbon-degrading bacteria 7 1.3.2. Pathways of aerobic hydrocarbon biodegradation 9 1.3.3. The possible role of cyanobacteria in petroleum-degrading 10 microbial communities 1.3.4. Biodegradation of hydrocarbons by anaerobic bacteria 11 1.4. Factors that influence biodegradation of crude oil in the 13 environment 1.5. Quantification of oil biodegradation 16 A.2. Nitrogen fixation – a brief literature overview 18 A.3. Objectives of the present work 22 B. Results and Discussion – an overview 23 1. Establishment of stratified microbial communities on oil- 23 contaminated marine sediments in experimental microcosms 2. Quantification of petroleum biodegradation on marine sediments in 27 experimental microcosm 2.1. Quantification of oil degradation via CO2 analysis 27 2.2.