Effect of Liquid Contamination on Hermeticity and Seal Strength of Flexible Pouches with LLDPE Sealant PREFACE API 2015
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Condiments – the Power and Potential of Packaging
White Paper Condiments – the power and potential of packaging More than just a sauce Condiments and sauces can be seen on dinner tables A brief walk through any food store shows just how popular across the globe. Although often overlooked, they can be condiments have become over the years. Much time and key in adding colour, texture, taste and flavour to what is investment is given to attracting the attention of shoppers being consumed. However, the actual taste experience is in the condiments aisle and keeping them coming back for often determined as much by the packaging and branding more. Despite its significant size, the global condiments of the product as by its contents1. With packaging playing a market is projected to continue to expand, reaching USD key role in customer satisfaction and purchasing decisions2, 98.3 billion by 2024 and growing at a significant CAGR of what can manufacturers learn from consumer purchasing 5.4% during the forecast period, 2019–2024.3 behaviour and how has packaging evolved to cope with the many demands now being placed on it? The psychology of condiment packaging With competition fierce for shelf space, condiments A growing market producers have had to get ever-more creative in how Since salt and vinegar were first used to enhance the they attract shoppers to their products. Here are some flavour of foods in ancient times, people have been hooked of the ways that psychology plays a part in condiments on condiments. From the Roman practice of crushing the packaging design. innards of various fish and fermenting them in salt to create garum, to today’s brightly coloured ketchups and mustards, Capturing consumer attention: a flourishing industry has grown. -

Food Packaging Technology
FOOD PACKAGING TECHNOLOGY Edited by RICHARD COLES Consultant in Food Packaging, London DEREK MCDOWELL Head of Supply and Packaging Division Loughry College, Northern Ireland and MARK J. KIRWAN Consultant in Packaging Technology London Blackwell Publishing © 2003 by Blackwell Publishing Ltd Trademark Notice: Product or corporate names may be trademarks or registered Editorial Offices: trademarks, and are used only for identification 9600 Garsington Road, Oxford OX4 2DQ and explanation, without intent to infringe. Tel: +44 (0) 1865 776868 108 Cowley Road, Oxford OX4 1JF, UK First published 2003 Tel: +44 (0) 1865 791100 Blackwell Munksgaard, 1 Rosenørns Allè, Library of Congress Cataloging in P.O. Box 227, DK-1502 Copenhagen V, Publication Data Denmark A catalog record for this title is available Tel: +45 77 33 33 33 from the Library of Congress Blackwell Publishing Asia Pty Ltd, 550 Swanston Street, Carlton South, British Library Cataloguing in Victoria 3053, Australia Publication Data Tel: +61 (0)3 9347 0300 A catalogue record for this title is available Blackwell Publishing, 10 rue Casimir from the British Library Delavigne, 75006 Paris, France ISBN 1–84127–221–3 Tel: +33 1 53 10 33 10 Originated as Sheffield Academic Press Published in the USA and Canada (only) by Set in 10.5/12pt Times CRC Press LLC by Integra Software Services Pvt Ltd, 2000 Corporate Blvd., N.W. Pondicherry, India Boca Raton, FL 33431, USA Printed and bound in Great Britain, Orders from the USA and Canada (only) to using acid-free paper by CRC Press LLC MPG Books Ltd, Bodmin, Cornwall USA and Canada only: For further information on ISBN 0–8493–9788–X Blackwell Publishing, visit our website: The right of the Author to be identified as the www.blackwellpublishing.com Author of this Work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. -

Pres-On Cap Liners & Seals
Pres-On Cap Liners & Seals OUR CAP LINERS AND SEALS MEET THE Pres-On cap liners and seals are found, hard at work, under UNIQUE PACKAGING REQUIREMENTS the lids of the world’s most popular consumer products OF A WIDE RANGE OF CONSUMER providing reliable protection against leaks, assisting in preserving PRODUCT CATEGORIES: freshness and authenticating product integrity, and aiding in preventing product contamination and strengthening consumer Automotive Personal Care confidence, while offering additional branding opportunities, and meeting specialized packaging needs. Beverage Beauty Chemical Pharmaceutical Converting Capabilities: Services: Food Nutraceutical • Rotary die-cutting • Cap liner extraction / insertion • Punch press die-cutting • Cap / container evaluations AND MORE! • Slitting • Custom design 2600 E. 107th Street | Bolingbrook, Illinois 60440 | TOLL FREE: 800.323.7467 | FAX: 630.628.8025 | [email protected] | PresOn.com Pres-On Cap Liners & Seals Pres-On excels in creating custom cap liners possessing a wide range of properties, able to satisfy challenging packaging requirements. Our heat induction foil seals, pressure seals, and foam liners are all available in diameters of 15mm to 125mm. Many of our materials can be custom die-cut into washers and pull tab seals, as well as custom shapes and sizes. In order to confidently produce the highest quality liners and seals, Pres-On only selects FDA approved materials from the industry’s leading material manufacturers including: JS Plastics, SanCap, Selig, Tech Seal, Tekniplex and -

POULTRY Packaging Solutions EN Poultry
Global Packaging Global Packaging POULTRY Packaging Solutions EN Poultry Specialists in Poultry Global Offer The poultry sector requires flexible packing solutions that ULMA is the only supplier capable of offering a wide range ranges from cost effective hygienic packaging to complex of solutions for the poultry sector. Complete solutions that packaging systems ensuring excellent presentation and start from handling and loading of the product to the final extended shelf-life. crate packing and palletizing. Only ULMA offers packaging solutions from five different packaging systems: Stretch film, ULMA is aware that the success of its clients depends on Horizontal Flow Pack (HFFS), Thermoforming, Traysealing the quality of their products and the protective packaging. and Vertical Flow Pack (VFFS). We draw from more than 50 years of experience developing packaging equipment to guarantee our clients the highest quality solutions for commercial success of your products and brands. Whole bird in-tray Packaging solutions designed for the packing of whole bird (chicken, turkey, duck and quail) placed on preformed trays and wrapped using flexible plastic film. The packing is achieved by a variety of ULMA automatic and/or semi-automatic wrapping machines. The different packaging solutions depend on the type of hermetic seal required, production levels, product’s shelf-life and the required presentation. ® SF - PVC SF - SSD ® FP - BDF Cost-effective packaging in stretch film. TS - MAP Packed in modified atmosphere (MAP) to increase the shelf-life of the product. ® FP - SES FP - SHRINK Wide range of films: • Stretch. • Stretch and shrink. • Barrier. • Barrier and shrink. MASTERBAG Whole bird without trays Packaging solutions designed for the packing of whole bird (chicken, turkey, duck and quail) where wrapping is required using plastic film without trays. -

Willingness to Pay for Hermetic Grain Storage Bags in Malawi
Willingness to Pay for Hermetic Grain Storage Bags in Malawi W.A. Masters¹; G. Guevara Alvarez² 1: Tufts University, Friedman School of Nutrition, United States of America, 2: Tufts University, Friedman School of Nutrition Science and Policy, United States of America Corresponding author email: [email protected] Abstract: This study estimates willingness to pay (WTP) for hermetic grain storage bags in a sample of 116 very low- income farmers, about half of whom had attended bag-use demonstrations designed to demonstrate how these bags prevent damage from mold and insects. WTP was measured using Becker-DeGroot-Marschak (BDM) auctions, accompanied by a survey regarding respondents’ education, household wealth and knowledge about aflatoxins that are released when mold is allowed to grow on the grain. We found a mean WTP of 311 Kwacha ($0.42) for one bag, well below the market price around 750 Kwacha, and no significant association between a respondent’s WTP and their attendance at bag-use demonstrations, aflatoxin knowledge or education and wealth. At current market prices, we found no evidence that these bags would be commercially marketable in these communities, even after bag-use demonstrations. A systematic review of the literature suggests that commercial sales of hermetic bags may be possible for buyers in areas of less extreme poverty, where households are more able to make such investments in pursuit of longer-term payoffs in food safety and storage. Acknowledegment: This report was funded by the American people through the United States Agency for International Development (USAID) via Cooperative Agreements AID-FFP-A-14-00006 and AID-OAA-A- 15-00019 to Catholic Relief Services (CRS) for the UBALE project. -

Vacuum Packaging
Packaging as it Relates to Core Storage and Preservation What follows is an attempt to synthesize current information from a wide variety of sources into a useful guide to methods of core storage and preservation. It must be stressed that the focus of the packaging industry is twofold. The first is the relatively short term preservation of, mostly, food and medical products. The second is packaging for protection and display purposes. Clearly, neither of these industries address the specific needs and requirements of IODP, so we must be creative and eclectic in our selection of the most suitable products for our application. Moreover, we must be actively involved in the research, testing and development of new products and methods by partnership/involvement with other institutions, so that IODP can be at the forefront of this technology as befits our ethos. Section 1 - Vacuum Packaging Vacuum packing (or vacuum sealing) is a form of packaging that involves the removal of air (and sometimes its replacement) from a pouch or plastic container. Vacuum packaging provides several benefits: protection against dehydration; barrier against air or moisture; tamper evident protection; compressed packaging for fragmented cores; protection from dust and moisture. Types of Vacuum Sealing Equipment Non Chamber Vacuum Sealers These units vacuum and seal the pouches externally. Some units also come equipped with special external nozzles to allow for the vacuum packaging using plastic containers. Non Chamber Vacuum sealers are meant for low volumes, and are suitable for vacuum sealing products that have little or no moisture. However some units do have special collectors for products that have excess moisture. -
![United States Patent (10) Patent N0.: US 7,114,312 B2 Coppeta Et A]](https://docslib.b-cdn.net/cover/3194/united-states-patent-10-patent-n0-us-7-114-312-b2-coppeta-et-a-983194.webp)
United States Patent (10) Patent N0.: US 7,114,312 B2 Coppeta Et A]
US007114312B2 (12) United States Patent (10) Patent N0.: US 7,114,312 B2 Coppeta et a]. (45) Date of Patent: Oct. 3, 2006 (54) LOW TEMPERATURE METHODS FOR 4,375,127 A * 3/1983 Elkins et a1. ............ .. 29/623.5 HERMETICALLY SEALING RESERVOIR 5,477,009 A * 12/1995 Brendecke et a1. ...... .. 174/52.3 DEVICES 5,797,898 A 8/1998 Santini, Jr. et a1. 6,114,658 A 9/2000 Roth et a1. (75) Inventors: Jonathan R. Coppeta, Windham, NH 6’123’861 A 9/2000 Sannm’ Jr‘ et a1‘ _ . 6,194,789 B1 2/2001 Zhou (US), Scott A. Uhland, Roslmdale, MA 6 221024 Bl 4/2001 M. _ . , , lesel (US), Benjamin F. Pollto, FlagstaiT, AZ 6,232,150 B1 50001 Lin et al‘ (US); Norman F- ShePRaYFL Jr» 6,237,398 B1 5/2001 Porat et a1. Bedford, MA (US); Chrlstlna M- 6,249,329 B1 * 6/2001 Dabral et a1. ............... .. 349/73 Feakes, Brighton, MA (US); Douglas 6,474,879 B1 11/2002 Warnes et a1. B. Snell, Amesbury, MA (US); John T. 6,491,666 B1 12/2002 Santini, Jr. et a1. santini, Jr" North chelmsfords MA 6,527,762 B1 3/2003 Santini, Jr. et al. (US) (Continued) (73) Assignee: MicroCHIPS, Inc., Bedford, MA (US) FOREIGN PATENT DOCUMENTS ( * ) Notice: Subject to any disclaimer, the term of this go W0 ; :1 i 1;; patent is extended or adjusted under 35 U30 1540)) by 10 days- OTHER PUBLICATIONS (21) App1_ No; 10/894,265 http:/WWW.qlpkg.com/product.html (Nov. 15, 2004). _ Primary ExamineriStephen F. -

Hermetic Feedthroughs Safeguard Mission-Critical Electronics
WHITE PAPER Hermetic Feedthroughs Safeguard Mission-Critical Electronics Choosing the right hermetic sealing technology for sealed electrical packages keeps vital applications up and running. From automotive electronic control units to weather satellites and defense systems, electronics failures can bring communications and critical functions to a sudden halt. Experts specializing in electronics failure analysis can list a host of reasons for various malfunctions, but one of the most common causes is simple moisture. Because water and electronics do not mix well, several strategies exist to protect mission-critical components from moisture and condensation. One of the most successful methods of protecting key components from water hazards involves hermetically sealed electronics cavities—enabled by moisture-blocking component assemblies such as hermetic feedthrough D-sub sealed in customer furnished enclosure for use in military flight application. technology. Sealed cavities are especially important in mission- feedthrough that will keep moisture from wicking critical applications such as those found in military into the electronics cavity while allowing power and and defense settings including power electronics signal conductors to enter. for missiles aboard ships and submarines or those in service aboard high altitude flights. Laser systems, IDENTIFYING WATER VAPOR AND CONDENSATION commercial and private avionics GPS units, high SOURCES voltage power generation and distribution networks, underwater pumps and automotive charging Electronics failures due to water vapor and stations represent other important applications condensation can occur in a number of ways. where electronics need to be protected from water Among the most common are corrosion of solder vapor and condensation. joints, shorts caused by water droplets, and stress corrosion cracking of seals and electrical interface Reliable sealing involves two major considerations— areas. -

Virtual Showroom May 2019.Pub
90 13th Avenue, Unit 11 Ronkonkoma, NY 11779 Spotlight 2019 ! Validatable for Medical Pouches Phone: 631-588-7310 We are pleased to recommend APM will be exhibiting at EAST Toll Free: 800-637-8808 our recently engineered Speed and versatility for Validatable Fax: 631-467-4690 Vertical Conveyorized Band handle cut-out bags! Conveyorized Model PACK in New York, June 11-13 E-mail: Sealer for Handle Pouches Medical Pouch Sealer is and PACK EXPO International [email protected] with Recloseable Zippers. This USDA Approved Rotary supplied with verification Website: ports for process validation in Las Vegas, September 23-25. www.allpackagingmachinery.com Band Sealer includes a 6” wide x 5’ long synchronized lower of pressure, speed, It is a great opportunity for you support conveyor and non- and temperature. to attend and speak with our motorized roller infeed. VCBS-3/8-DH-10-Validate VCBSDM-ET-1/4-6x5 WOLCB experienced engineers to learn floor model renders a ¼” wide Complete, visible validation seal without trim. Extended for medical pouches. Single line embossing coder available. more about our pouch and bag sealing throat allows seal to be 3/8” wide hermetic seal. Dual heating and dual cooling bars. placed between a handle cut- Includes a synchronized conveyor and self-contained air sealing equipment. Many differ- compressor. Seals Tyvek® pouches up to 40 feet per minute. out and a recloseable zipper. ent models are available for This sealer uses 1” wide heavy duty teflon bands as a standard diverse applications such as design. Adjustable height seal- ing assembly with crank han- meat, poultry, seafood, bakery, dle. -

NEPP ETW 2013: Hermeticity Leak Testing
NASA Electronic Parts and Packaging (NEPP) Hermeticity Task Overview NEPP Program Task 13-294: Hermeticity Correlation Study NASA MSFC/GSFC ES43 / Patrick McManus ES43-ERC / Kathy Pressnell GSFC-5620 / Lyudmyla Panashchenko www.nasa.gov June 11, 2013 AGENDA I. Introduction II. Overview of Hermeticity III. Hermeticity Correlation Study A. Helium Desorption Issue B. Instrument Correlation Study C. Leak Standard Development D. Test Method Optimization IV. Future Work 2 INTRODUCTION Hermeticity task is a collaborative effort between GSFC/MSFC to address the following: • Gain understanding of the influence of component part material on resultant leak rate data • Determine CHLD test equipment capability between NASA centers as well as correlation of test results with other equipment used for hermeticity testing (OLT, Krypton-85, IGA) • Design, fabricate, and test gross leak hermeticity standards • Provide input to DLA Land & Maritime to optimize hermeticity specifications based on the knowledge gained during correlation study, part testing, and research efforts 3 OVERVIEW “Why, When, How, and What” Of Hermeticity Testing • Fine and gross leak testing is used to determine the effectiveness of package seals in microelectronic packages. Damaged or defective seals and feedthroughs allow ambient air/water vapor to enter the internal cavity of the device which can result in internal corrosion leading to device failures. • Testing may be performed just after sealing process, but usually performed during screening/qualification. Sometimes performed as part of a DPA or failure analysis. • Testing is performed in accordance with MIL-STD-883, Test Method 1014 for hybrids/microcircuits and MIL-STD-750 for 1071 for discrete semiconductor devices • Three systems are used to non-destructively test: CHLD, KR-85, OLT • CHLD, Kr-85 systems use back pressurization of a tracer gas to enter existing leak paths. -

Glenair Hermetic Connector Products Helium Leak Rates and Testing
Hermetic Connectors Hermetic Introduction to Glenair Hermetic Connector Products Helium Leak Rates and Testing A Hermeticity Hermeticity is defined as “the state or condition of being airtight”. Sophisticated military electronics enclosures can experience electrical failure from ingress of moisture. System engineers can design the enclosure to withstand exposure to moisture and condensation by using “moisture-hardened” components and conformal coatings, but often the most practical approach is to install hermetically sealed electrical I/O connectors. Glass-to-metal seals provide assurance that, over the life of the enclosure, the accumulated amount of water vapor inside the box will not exceed the amount necessary to form condensation. Other applications for Micro-D hermetic connectors include vacuum chambers, cryogenics, and enclosures filled with inert gas. Hermetic Testing All hermetic connectors are 100% tested prior to shipment. A helium leak test is performed to certify the hermetic seal. This test is conducted by inducing a 1 ATM vacuum on one side of the connector. Helium gas is released on the other side, and a mass spectrometer "counts" the number of helium molecules that penetrate the connector seal. Helium leak testing takes advantage of the small size of a helium molecule compared to air or water vapor. Helium is inert, rare in our atmosphere, and is easy to detect with a mass spectrometer. Helium Leak Rates Std cc/sec Approximate Approximate Bubble Equivalent 1 x 10-1 1 cc/10 sec 1 x 10-2 1 cc/100 sec 1 x 10-3 3 cc/hour 1 x 10-4 1 cc/3 hours 1 x 10-5 1 cc/24 hours 1 x 10-6 1 cc/2 weeks 1 x 10-7 3 cc/year 1 x 10-8 1 cc/3 year 1 x 10-9 1 cc/30 years 1 x 10-11 1 cc/3000 years Close up of gas tube assembly undergoing helium leak test at the Glenair Factory in Glendale, CA © 2013 Glenair, Inc. -
Span and Offset
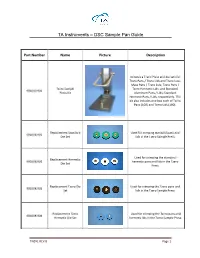
TA Instruments – DSC Sample Pan Guide Part Number Name Picture Description Includes a Tzero Press and die sets for Tzero Pans / Tzero Lids and Tzero Low- Mass Pans / Tzero Lids; Tzero Pans / Tzero Sample Tzero Hermetic Lids; and Standard 901600.901 Press Kit Aluminum Pans / Lids; Standard Hermetic Pans / Lids, respectively. The kit also includes one box each of Tzero Pans (100) and Tzero Lids (100). Replacement Standard Used for crimping standard pans and 901608.901 Die Set lids in the Tzero Sample Press Used for crimping the standard Replacement Hermetic 901608.902 hermetic pans and lids in the Tzero Die Set Press Replacement Tzero Die Used for crimping the Tzero pans and 901608.903 Set lids in the Tzero Sample Press Replacement Tzero Used for crimping the Tzero pans and 901608.904 Hermetic Die Set hermetic lids in the Tzero Sample Press. TN071 REV B Page 1 An optional die set for use with the Tzero Press in sealing of a sample in the TA Instruments Standard Series DSC High Volume Die High Volume Stainless Steel pans and 901608.905 Set lids. The set consists of a lower and upper die and is designed to encapsulate various pressure sensitive materials. Lightweight aluminum pans recommended for high performance analysis of small samples (< 10 mg). Tzero Low-Mass Pans The pans have uniformly flat bottoms (pkg. of 100) designed for very good contact with 901670.901 the Tzero cell sensors. When sealed Temperature Range: with the Tzero Lid (P/N: 901671.901), -180°C to 600°C the internal volume is 10 µL.