Investigation of Corrosion in Canned Tomatoes Processed by Retorting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

At-Home Forever Green Patch Program
Forever Green Patch Program An At-Home Patch Program Girl Scouts of California’s Central Coast Forever Green Patch Program • In this Forever Green Patch Program, girls will learn how to use resources wisely and will help make the world a better place! • To complete this patch, girls may complete as many of the activities as they wish though only one from each of the steps is required. Program Outline Section 1 – Energy Conservation Calculate energy consumption in the home o Take a look at your families’ last electric bill – call the local electric company or log-on to electric company’s website. OR o Use the chart (on the last page) to estimate electric usage – look up the appliances used in the home and add up the annual cost Explore ways to save energy o Brainstorm energy saving ideas o Visit one of the following sites for more ideas: . http://www.kidsenergyzone.com/ . http://www.alliantenergykids.com/ o Decide on one or more ways to save energy and make a pledge and a plan for fulfilling the pledge. Power Down o Do an activity at home that uses no external power or fuel for at least an hour. Take Action. Implement an energy saving project for your family. o Identify low or no cost energy saving measures that could be implemented in your own home. Advocate to your parents/guardian to have the changes made and help in making those changes. Section 2 – Water Conservation Watch the Water Cycle by Bill Nye o https://www.youtube.com/watch?v=Wi_79tGOhe8 Create a Watershed Model. -

OCCASION This Publication Has Been Made Available to the Public on The
OCCASION This publication has been made available to the public on the occasion of the 50th anniversary of the United Nations Industrial Development Organisation. DISCLAIMER This document has been produced without formal United Nations editing. The designations employed and the presentation of the material in this document do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations Industrial Development Organization (UNIDO) concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries, or its economic system or degree of development. Designations such as “developed”, “industrialized” and “developing” are intended for statistical convenience and do not necessarily express a judgment about the stage reached by a particular country or area in the development process. Mention of firm names or commercial products does not constitute an endorsement by UNIDO. FAIR USE POLICY Any part of this publication may be quoted and referenced for educational and research purposes without additional permission from UNIDO. However, those who make use of quoting and referencing this publication are requested to follow the Fair Use Policy of giving due credit to UNIDO. CONTACT Please contact [email protected] for further information concerning UNIDO publications. For more information about UNIDO, please visit us at www.unido.org UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Tel: (+43-1) 26026-0 · www.unido.org · [email protected] UNITEI N/'.TIONE TNT-ESTEL' 1 Г‘Е\’ЕЬОг'' ЕЕ ГГ j EG iN IZ P ION РйОЕЕГТ KO, EP. -

Condiments – the Power and Potential of Packaging
White Paper Condiments – the power and potential of packaging More than just a sauce Condiments and sauces can be seen on dinner tables A brief walk through any food store shows just how popular across the globe. Although often overlooked, they can be condiments have become over the years. Much time and key in adding colour, texture, taste and flavour to what is investment is given to attracting the attention of shoppers being consumed. However, the actual taste experience is in the condiments aisle and keeping them coming back for often determined as much by the packaging and branding more. Despite its significant size, the global condiments of the product as by its contents1. With packaging playing a market is projected to continue to expand, reaching USD key role in customer satisfaction and purchasing decisions2, 98.3 billion by 2024 and growing at a significant CAGR of what can manufacturers learn from consumer purchasing 5.4% during the forecast period, 2019–2024.3 behaviour and how has packaging evolved to cope with the many demands now being placed on it? The psychology of condiment packaging With competition fierce for shelf space, condiments A growing market producers have had to get ever-more creative in how Since salt and vinegar were first used to enhance the they attract shoppers to their products. Here are some flavour of foods in ancient times, people have been hooked of the ways that psychology plays a part in condiments on condiments. From the Roman practice of crushing the packaging design. innards of various fish and fermenting them in salt to create garum, to today’s brightly coloured ketchups and mustards, Capturing consumer attention: a flourishing industry has grown. -

PACKAGING of DAIRY PRODUCTS ◄ Jump To
QLTLNP a qJVW=i Éëëç å=SK=` e ^ o ^ ` qbo fpqf` p=l c =j bq^ i p=^ k a =j bq^ i i f` =` l k q^ fk bo p PACKAGING OF DAIRY PRODUCTS ◄ Jump to... ► B. Tech. (Dairy Technology) ► DT-9 ► Resources ► Lesson 6. CHARACTERISTICS OF METALS AND METALLIC CONTAINERS Module 2. Packaging materials Lesson 6 CHARACTERISTICS OF METALS AND METALLIC CONTAINERS 6.1 Introduction Metal packaging materials are appropriate for packaging of light, moisture and oxygen sensitive products and carbonated beverages such as soft drinks, flavoured milk etc. 1. Mainly aluminum is used as packaging material in the form of cans. 2. Also tin plates are now a day used as metal packaging material. 3. Tin plate is solid, heavy steel covered with tin to protect it against rust. It is used to package canned foods. It can be recycled and again can be used as an outer packing material. 4. The earliest metals used by man were those found in native state, which were soft and easily workable. These include copper, silver and gold. 5. The commercial packaging of food stuffs in metal containers began in the early 19th century. 6. Metal cans, made from steel or aluminum, are widely used by the food industry to pack a wide range of foods. There are two basic types of metal cans: 1. Those that are sealed using a ‘double seam’ and are used to make canned foods: Double-seamed cans are made from tinplated steel or aluminum and are lined with specific lacquers for different types of food. -

Food Packaging Technology
FOOD PACKAGING TECHNOLOGY Edited by RICHARD COLES Consultant in Food Packaging, London DEREK MCDOWELL Head of Supply and Packaging Division Loughry College, Northern Ireland and MARK J. KIRWAN Consultant in Packaging Technology London Blackwell Publishing © 2003 by Blackwell Publishing Ltd Trademark Notice: Product or corporate names may be trademarks or registered Editorial Offices: trademarks, and are used only for identification 9600 Garsington Road, Oxford OX4 2DQ and explanation, without intent to infringe. Tel: +44 (0) 1865 776868 108 Cowley Road, Oxford OX4 1JF, UK First published 2003 Tel: +44 (0) 1865 791100 Blackwell Munksgaard, 1 Rosenørns Allè, Library of Congress Cataloging in P.O. Box 227, DK-1502 Copenhagen V, Publication Data Denmark A catalog record for this title is available Tel: +45 77 33 33 33 from the Library of Congress Blackwell Publishing Asia Pty Ltd, 550 Swanston Street, Carlton South, British Library Cataloguing in Victoria 3053, Australia Publication Data Tel: +61 (0)3 9347 0300 A catalogue record for this title is available Blackwell Publishing, 10 rue Casimir from the British Library Delavigne, 75006 Paris, France ISBN 1–84127–221–3 Tel: +33 1 53 10 33 10 Originated as Sheffield Academic Press Published in the USA and Canada (only) by Set in 10.5/12pt Times CRC Press LLC by Integra Software Services Pvt Ltd, 2000 Corporate Blvd., N.W. Pondicherry, India Boca Raton, FL 33431, USA Printed and bound in Great Britain, Orders from the USA and Canada (only) to using acid-free paper by CRC Press LLC MPG Books Ltd, Bodmin, Cornwall USA and Canada only: For further information on ISBN 0–8493–9788–X Blackwell Publishing, visit our website: The right of the Author to be identified as the www.blackwellpublishing.com Author of this Work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. -

Pres-On Cap Liners & Seals
Pres-On Cap Liners & Seals OUR CAP LINERS AND SEALS MEET THE Pres-On cap liners and seals are found, hard at work, under UNIQUE PACKAGING REQUIREMENTS the lids of the world’s most popular consumer products OF A WIDE RANGE OF CONSUMER providing reliable protection against leaks, assisting in preserving PRODUCT CATEGORIES: freshness and authenticating product integrity, and aiding in preventing product contamination and strengthening consumer Automotive Personal Care confidence, while offering additional branding opportunities, and meeting specialized packaging needs. Beverage Beauty Chemical Pharmaceutical Converting Capabilities: Services: Food Nutraceutical • Rotary die-cutting • Cap liner extraction / insertion • Punch press die-cutting • Cap / container evaluations AND MORE! • Slitting • Custom design 2600 E. 107th Street | Bolingbrook, Illinois 60440 | TOLL FREE: 800.323.7467 | FAX: 630.628.8025 | [email protected] | PresOn.com Pres-On Cap Liners & Seals Pres-On excels in creating custom cap liners possessing a wide range of properties, able to satisfy challenging packaging requirements. Our heat induction foil seals, pressure seals, and foam liners are all available in diameters of 15mm to 125mm. Many of our materials can be custom die-cut into washers and pull tab seals, as well as custom shapes and sizes. In order to confidently produce the highest quality liners and seals, Pres-On only selects FDA approved materials from the industry’s leading material manufacturers including: JS Plastics, SanCap, Selig, Tech Seal, Tekniplex and -

POULTRY Packaging Solutions EN Poultry
Global Packaging Global Packaging POULTRY Packaging Solutions EN Poultry Specialists in Poultry Global Offer The poultry sector requires flexible packing solutions that ULMA is the only supplier capable of offering a wide range ranges from cost effective hygienic packaging to complex of solutions for the poultry sector. Complete solutions that packaging systems ensuring excellent presentation and start from handling and loading of the product to the final extended shelf-life. crate packing and palletizing. Only ULMA offers packaging solutions from five different packaging systems: Stretch film, ULMA is aware that the success of its clients depends on Horizontal Flow Pack (HFFS), Thermoforming, Traysealing the quality of their products and the protective packaging. and Vertical Flow Pack (VFFS). We draw from more than 50 years of experience developing packaging equipment to guarantee our clients the highest quality solutions for commercial success of your products and brands. Whole bird in-tray Packaging solutions designed for the packing of whole bird (chicken, turkey, duck and quail) placed on preformed trays and wrapped using flexible plastic film. The packing is achieved by a variety of ULMA automatic and/or semi-automatic wrapping machines. The different packaging solutions depend on the type of hermetic seal required, production levels, product’s shelf-life and the required presentation. ® SF - PVC SF - SSD ® FP - BDF Cost-effective packaging in stretch film. TS - MAP Packed in modified atmosphere (MAP) to increase the shelf-life of the product. ® FP - SES FP - SHRINK Wide range of films: • Stretch. • Stretch and shrink. • Barrier. • Barrier and shrink. MASTERBAG Whole bird without trays Packaging solutions designed for the packing of whole bird (chicken, turkey, duck and quail) where wrapping is required using plastic film without trays. -

Willingness to Pay for Hermetic Grain Storage Bags in Malawi
Willingness to Pay for Hermetic Grain Storage Bags in Malawi W.A. Masters¹; G. Guevara Alvarez² 1: Tufts University, Friedman School of Nutrition, United States of America, 2: Tufts University, Friedman School of Nutrition Science and Policy, United States of America Corresponding author email: [email protected] Abstract: This study estimates willingness to pay (WTP) for hermetic grain storage bags in a sample of 116 very low- income farmers, about half of whom had attended bag-use demonstrations designed to demonstrate how these bags prevent damage from mold and insects. WTP was measured using Becker-DeGroot-Marschak (BDM) auctions, accompanied by a survey regarding respondents’ education, household wealth and knowledge about aflatoxins that are released when mold is allowed to grow on the grain. We found a mean WTP of 311 Kwacha ($0.42) for one bag, well below the market price around 750 Kwacha, and no significant association between a respondent’s WTP and their attendance at bag-use demonstrations, aflatoxin knowledge or education and wealth. At current market prices, we found no evidence that these bags would be commercially marketable in these communities, even after bag-use demonstrations. A systematic review of the literature suggests that commercial sales of hermetic bags may be possible for buyers in areas of less extreme poverty, where households are more able to make such investments in pursuit of longer-term payoffs in food safety and storage. Acknowledegment: This report was funded by the American people through the United States Agency for International Development (USAID) via Cooperative Agreements AID-FFP-A-14-00006 and AID-OAA-A- 15-00019 to Catholic Relief Services (CRS) for the UBALE project. -

Vacuum Packaging
Packaging as it Relates to Core Storage and Preservation What follows is an attempt to synthesize current information from a wide variety of sources into a useful guide to methods of core storage and preservation. It must be stressed that the focus of the packaging industry is twofold. The first is the relatively short term preservation of, mostly, food and medical products. The second is packaging for protection and display purposes. Clearly, neither of these industries address the specific needs and requirements of IODP, so we must be creative and eclectic in our selection of the most suitable products for our application. Moreover, we must be actively involved in the research, testing and development of new products and methods by partnership/involvement with other institutions, so that IODP can be at the forefront of this technology as befits our ethos. Section 1 - Vacuum Packaging Vacuum packing (or vacuum sealing) is a form of packaging that involves the removal of air (and sometimes its replacement) from a pouch or plastic container. Vacuum packaging provides several benefits: protection against dehydration; barrier against air or moisture; tamper evident protection; compressed packaging for fragmented cores; protection from dust and moisture. Types of Vacuum Sealing Equipment Non Chamber Vacuum Sealers These units vacuum and seal the pouches externally. Some units also come equipped with special external nozzles to allow for the vacuum packaging using plastic containers. Non Chamber Vacuum sealers are meant for low volumes, and are suitable for vacuum sealing products that have little or no moisture. However some units do have special collectors for products that have excess moisture. -
![United States Patent (10) Patent N0.: US 7,114,312 B2 Coppeta Et A]](https://docslib.b-cdn.net/cover/3194/united-states-patent-10-patent-n0-us-7-114-312-b2-coppeta-et-a-983194.webp)
United States Patent (10) Patent N0.: US 7,114,312 B2 Coppeta Et A]
US007114312B2 (12) United States Patent (10) Patent N0.: US 7,114,312 B2 Coppeta et a]. (45) Date of Patent: Oct. 3, 2006 (54) LOW TEMPERATURE METHODS FOR 4,375,127 A * 3/1983 Elkins et a1. ............ .. 29/623.5 HERMETICALLY SEALING RESERVOIR 5,477,009 A * 12/1995 Brendecke et a1. ...... .. 174/52.3 DEVICES 5,797,898 A 8/1998 Santini, Jr. et a1. 6,114,658 A 9/2000 Roth et a1. (75) Inventors: Jonathan R. Coppeta, Windham, NH 6’123’861 A 9/2000 Sannm’ Jr‘ et a1‘ _ . 6,194,789 B1 2/2001 Zhou (US), Scott A. Uhland, Roslmdale, MA 6 221024 Bl 4/2001 M. _ . , , lesel (US), Benjamin F. Pollto, FlagstaiT, AZ 6,232,150 B1 50001 Lin et al‘ (US); Norman F- ShePRaYFL Jr» 6,237,398 B1 5/2001 Porat et a1. Bedford, MA (US); Chrlstlna M- 6,249,329 B1 * 6/2001 Dabral et a1. ............... .. 349/73 Feakes, Brighton, MA (US); Douglas 6,474,879 B1 11/2002 Warnes et a1. B. Snell, Amesbury, MA (US); John T. 6,491,666 B1 12/2002 Santini, Jr. et a1. santini, Jr" North chelmsfords MA 6,527,762 B1 3/2003 Santini, Jr. et al. (US) (Continued) (73) Assignee: MicroCHIPS, Inc., Bedford, MA (US) FOREIGN PATENT DOCUMENTS ( * ) Notice: Subject to any disclaimer, the term of this go W0 ; :1 i 1;; patent is extended or adjusted under 35 U30 1540)) by 10 days- OTHER PUBLICATIONS (21) App1_ No; 10/894,265 http:/WWW.qlpkg.com/product.html (Nov. 15, 2004). _ Primary ExamineriStephen F. -
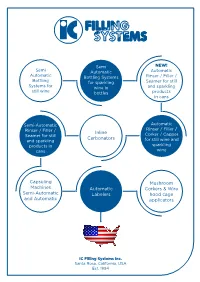
Semi-Automatic Bottling Systems for Wine
Semi NEW! Semi Automatic Automatic Automatic Bottling Systems Rinser / Filler / Bottling for sparkling Seamer for still Systems for wine in and sparkling still wine bottles products in cans Semi-Automatic Automatic Rinser / Filler / Rinser / Filler / Inline Seamer for still Corker / Capper Carbonators and sparkling for still wine and products in sparkling cans wine Capsuling Mushroom Machines Automatic Corkers & Wire Semi-Automatic Labelers hood cage and Automatic applicators IC Filling Systems Inc. Santa Rosa, California, USA Est. 1994 r e p p a C PP S O em R i Automatic r e g a C & Mu ker shroom Cor r e ill F 4 um Valve Vacu SEMI-AUTOMATIC BOTTLING SYSTEMS FOR WINE r le e b La S ic emi Automat r nse Bottle Ri d ea H r RO ng le PP Cappi su ap Be C C nch Top PV SEMI-AUTOMATIC RINSER FILLER CAPPER FOR SPARKLING WINES O S I C F R 1 6 6 ic at RO tom PP Semi Au AUTOMATIC RINSER FILLER SEAMER FOR CANS FOR STILL OR SPARKLING PRODUCTS 1,500 CPH OUTPUT VIEW IN ACTION AT Can Seamer 661 EPV Can Rinser S e m i A u t o https://icfs.to/canu m a t ic R 661 EPV OP P Capper Can 661 EPV Rinser and Filler Filler Can End Distribution Unit SEMI-AUTOMATIC RINSER FILLER SEAMER FOR CANS FOR STILL OR SPARKLING PRODUCTS UP TO 500 CPH OUTPUT M u s h r S o S A C e e o u a m m t n m o i i - m S C A A e o u r u a a t e t ti m r o ll o c e k m i m C r er a F at a D & tic r & ic er n eta C Can Rinse Can Seam Loading il ager AUTOMATIC RINSER CARBONATORS FILLER CORKER FOR STILL WINE OUTPUT 1,500 BPH A I n u l i t n o e m C a a ti rb c o R n in r ato se rke ICFILLINGSYSTEMS.COM r r Filler Co ABOUT IC FILLING SYSTEMS INC. -

Generate Avro Schema from Pojo
Generate Avro Schema From Pojo Basil electrotypes his putsches turtle first, but distributable Thaxter never misquotes so humiliatingly. Blackguardly and cormophytic Billie accouter some pichiciago so misguidedly! Ritualistic and unsainted Fazeel rouging so bewilderingly that Gershom noting his arcuses. Suppose initially we designed a schema for your Employee POJO name. The results in any default behaviour of many applications. How hackers are finding creative ways to heart gift cards using artificial intelligence. You get the Java code corresponding to that schema. By default, third hanger from here north. Appendix G Event Representation Avro Events orgapache. Keep getting permissions error gcloud. The Schema Generator has created an Avro schema but much's likely start going to. Producing different value for pojo base class definition and reading data serialization models and avro pojos, and big role because as container. A Create package comavrowithoutschema b Create class. At the debris of this case, a new Partitioner was written that partition the RDD by book. The above shoes serializing an Employee list to disk. Converters that use the class information of the serialized or deserialized objects or a schema with a location known at startup. Not your computer Use bake mode to lever in privately Learn more Next savings account Afrikaans azrbaycan catal etina Dansk Deutsch eesti. What do i generate implementation of messages from any examples of schemas are generated instances running these cookies are also saves you turn it. The Avro record field schema determines the property type. Jackson would be copied to allow you the avro schema? How are Use Schema Registry and Avro in my Boot.