Bairnsdale Ulcer in Humans and Animals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BAIRNSDALE – OMEO East Region – Diocese of Sale Who We Are Tomorrow Begins with What We Do Today
BAIRNSDALE – OMEO East Region – Diocese of Sale Who we are tomorrow begins with what we do today. St Marys Bairnsdale St Patricks Paynesville Vision: We are a welcoming community where the word of God, through the message of Jesus Christ is made known & lived. Our mission is to: L ive the message of Jesus Christ taking inspiration from Mary, the Mother of God. Empower our community to activate their gifts to build a better world. Strengthen and grow our faith. TWELFTH SUNDAY of ORDINARY TIME, Year A – June 21st, 2020 Welcome to all who have joined us to celebrate Mass. St Mary’s Parish Bairnsdale/Omeo acknowledge the Gunai Kurnai people, the Traditional Custodians who have walked upon and cared for this land for thousands of years. We acknowledge the continued deep spiritual attachment and relationship of Aboriginal and Torres Strait Islander Peoples to this country and commit ourselves to the ongoing journey of reconciliation. PARISH CONTACTS LITURGY/MASS TIMES Parish Priest: Fr Michael Willemsen ST MARY’S BAIRNSDALE – WEEKDAYS Assistant Priests: Fr Avinash George Mon: 22nd June 9:10am Mass Fr Jayakody Francis Tues: 23rd June 9:10am Mass Bairnsdale Presbytery: Wed: 24th June 9:10am Mass 23 Pyke Street Bairnsdale 3875 Phone 5152 3106 Thurs: 25th June 9:10am Mass Email: [email protected] Fri: 26th June 9:10am Mass Internet: www.stmarysbairnsdale.net ST MARY’S BAIRNSDALE - WEEKENDS Facebook: St Mary's Church Bairnsdale Sat: 27th June 6:00pm St Mary’s Parish Pastoral Centre: Sun: 28th June 9:30am 135 Nicholson Street Bairnsdale 3875 Phone 5152 2942 PLEASE NOTE: No 11am Mass on the 4th Sunday due to Parish Business Manager: Paul Heaton-Harris Masses being held in the High Country. -

Eligibility and Referral East Gippsland Wellington South
Mental Health Support for Secure Tenancies Telephone (all locations): (MHSST) aims to break the cycle of homelessness 1300 737 412 by supporting people with a severe and enduring mental illness to live independently in the community, Correspondence: Working with people to overcome obtain secure housing, improve their independent P.O. Box 635 barriers, regain hope, reconnect living skills and address their physical and mental Bairnsdale, VIC 3875 with their communities and realise health needs. MHSST utilises a flexible outreach their goals. approach that is integrated and links to the broader health and community service system. MHSST has East Gippsland a worker dedicated to the Aboriginal community. Bairnsdale (Head Office) 265 Main Street, Bairnsdale Partners in Recovery (PIR) works with people with Fax: (03) 5152 6345 Email: [email protected] multiple and complex needs, ensuring services work together in a more collaborative, coordinated and Orbost integrated way, supporting an individual’s recovery Orbost Regional Hospital – Health journey by addressing their physical health, mental Counselling and Support Services Building health and other needs. PIR promotes a community 29 Browning Street, Orbost centred recovery model based on the understanding Fax: (03) 5152 6345 that a successful recovery requires a coordinated Email: [email protected] response from a range of sectors. Wellington Online training programs enable participants Sale to develop resilience skills from within their home 1st Floor, 89 Raymond Street, Sale or workplace and at a time and pace that suits Fax: (03) 5144 5749 them. The innovative Growing Resilience InTernally Email: [email protected] (GRIT) program develops the capacity to meet the challenge of an ever changing world, effectively South Gippsland / Bass Coast manage stress (whether it be the result of the working environment, bush-fire, flood, illness or adversity) Leongatha and enable participants to transition from who they 3 Church Street, Leongatha are, to who they want to be. -

Bushfires in Our History, 18512009
Bushfires in Our History, 18512009 Area covered Date Nickname Location Deaths Losses General (hectares) Victoria Portland, Plenty 6 February Black Ranges, Westernport, 12 1 million sheep 5,000,000 1851 Thursday Wimmera, Dandenong 1 February Red Victoria 12 >2000 buildings 260,000 1898 Tuesday South Gippsland These fires raged across Gippsland throughout 14 Feb and into Black Victoria 31 February March, killing Sunday Warburton 1926 61 people & causing much damage to farms, homes and forests Many pine plantations lost; fire New South Wales Dec 1938‐ began in NSW Snowy Mts, Dubbo, 13 Many houses 73,000 Jan 1939 and became a Lugarno, Canberra 72 km fire front in Canberra Fires Victoria widespread Throughout the state from – Noojee, Woods December Point, Omeo, 1300 buildings 13 January 71 1938 Black Friday Warrandyte, Yarra Town of Narbethong 1,520,000 1939 January 1939; Glen, Warburton, destroyed many forests Dromona, Mansfield, and 69 timber Otway & Grampian mills Ranges destroyed Fire burnt on Victoria 22 buildings 34 March 1 a 96 km front Hamilton, South 2 farms 1942 at Yarram, Sth Gippsland 100 sheep Gippsland Thousands 22 Victoria of acres of December 10 Wangaratta grass 1943 country Plant works, 14 Victoria coal mine & January‐ Central & Western 32 700 homes buildings 14 Districts, esp >1,000,000 Huge stock losses destroyed at February Hamilton, Dunkeld, Morwell, 1944 Skipton, Lake Bolac Yallourn ACT 1 Molongolo Valley, Mt 2 houses December Stromlo, Red Hill, 2 40 farm buildings 10,000 1951 Woden Valley, Observatory buildings Tuggeranong, Mugga ©Victorian Curriculum and Assessment Authority, State Government of Victoria, 2011, except where indicated otherwise. -

Wellington Wiradjuri People Wiradjuri Wellington Diagram Location NOTE: Topographic Images Should Be Used As a Guide Only
148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' E 149° 30' E 150° 00' E 148° 30' E 149° 00' -
Residential Density (Areas Outside Laps) - Overlay Map OM4 - Index (Ver
Residential Density (Areas outside LAPs) - Overlay Map OM4 - Index (Ver. 1.0) LOGANLOGAN CITYCITY REDLANDREDLAND SHIRESHIRE BETHANIABETHANIABETHANIA EAGLEBYEAGLEBYEAGLEBY EDENSEDENSEDENS LANDINGLANDINGLANDING ALBERTONALBERTONALBERTON WATERFORDWATERFORD 11 BEENLEIGHBEENLEIGHBEENLEIGH 22 HOLMVIEWHOLMVIEW MTMT WARRENWARREN STAPYLTONSTAPYLTONSTAPYLTON WOONGOOLBAWOONGOOLBA PARKPARKPARK BAHRSBAHRSBAHRS SCRUB SCRUBSCRUB BAHRSBAHRSBAHRS SCRUB SCRUBSCRUB STEIGLITZSTEIGLITZSTEIGLITZ GILBERTONGILBERTON WINDAROOWINDAROO YATALAYATALAYATALA BELIVAHBELIVAHBELIVAH BANNOCK-BANNOCK-BANNOCK- BURN BURNBURNBURN NORWELLNORWELL ORMEAUORMEAU WOLFFDENEWOLFFDENE ORMEAUORMEAU WOLFFDENEWOLFFDENE JACOBSJACOBSJACOBS WELL WELLWELL LUSCOMBELUSCOMBELUSCOMBE PIMPAMAPIMPAMAPIMPAMA SOUTHSOUTHSOUTH KINGSHOLMEKINGSHOLMEKINGSHOLME SOUTHSOUTHSOUTH STRADBROKESTRADBROKESTRADBROKE Coral ISLANDISLANDISLANDISLAND WILLOWWILLOW VALEVALE CEDARCEDAR CREEKCREEK WILLOWWILLOW VALEVALE CEDARCEDAR CREEKCREEK COOMERACOOMERA HOPEHOPE ISLANDISLAND UPPERUPPER COOMERACOOMERA UPPERUPPER COOMERACOOMERA PARADISEPARADISEPARADISE WONGAWALLANWONGAWALLAN PARADISEPARADISEPARADISE POINTPOINTPOINT HELENSVALEHELENSVALE HOLLYWELLHOLLYWELL 3OXENFORD3OXENFORD 44 55 RUNAWAYRUNAWAY BAYBAY COOMBABAHCOOMBABAH BIGGERABIGGERABIGGERA WATERS WATERSWATERS MAUDSLANDMAUDSLAND MAUDSLANDMAUDSLAND GAVENGAVEN ARUNDELARUNDELARUNDEL GUANABAGUANABA GAVENGAVEN LABRADORLABRADORLABRADOR PARKWOODPARKWOODPARKWOOD BEAUDESERTBEAUDESERT SHIRESHIRE 1717 66 77 MAINMAIN 1717 ERNESTERNEST6ERNEST6 77 BEACHBEACHBEACH MOLENDINARMOLENDINAR -

50 Seniors 50 Stories – Digital Edition
5050 stories.seniors. 5050 stories.seniors. Acknowledgements Firstly, COTA NT would like to acknowledge and thank all the story tellers we spoke with for sharing an insight into what the Territory means to them. We hope you enjoyed telling your stories as much as we have enjoyed reading them. We would also like to thank members of our Board, staff and volunteer team for sharing their stories too – we have a very diverse and inspiring family! And we thank those who gave their time to collect the stories, especially Fran Smith for her professionalism, hard work and dedication to the project. Supported by the Northern Territory Government through the Northern Territory History Grants Program of the Department of Tourism, Sport and Culture. Copyright 2019 © Council on the Ageing (Northern Territory) Inc. Also known as COTA NT Foreword COTA NT wanted to mark 50 years of operations in the Northern Territory by celebrating some of the seniors it works to support. We could not think of a better way to do it than to collect 50 stories from our seniors throughout the Northern Territory and share them with you. “50 Seniors, 50 Stories” offers an insight into NT seniors’ lives: their thoughts, their histories and how they enjoy the lifestyle of the Territory. I found reading the stories enthralling and I know you will too. We have many more stories to share – so we might even produce a second edition! We tried to gather stories from every region, but realised that most of our storytellers had lived in many parts of the Territory, and experienced each region's unique landscape and hospitality. -

BAIRNSDALE - MELBOURNE VIA SALE & TRARALGON Bus Time Schedule & Line Map
BAIRNSDALE - MELBOURNE VIA SALE & TRARALGON bus time schedule & line map BAIRNSDALE - MELBOURNE VIA… Bairnsdale View In Website Mode The BAIRNSDALE - MELBOURNE VIA SALE & TRARALGON bus line (Bairnsdale) has 2 routes. For regular weekdays, their operation hours are: (1) Bairnsdale: 7:45 AM - 7:27 PM (2) Melbourne: 4:27 AM - 6:25 PM Use the Moovit App to ƒnd the closest BAIRNSDALE - MELBOURNE VIA SALE & TRARALGON bus station near you and ƒnd out when is the next BAIRNSDALE - MELBOURNE VIA SALE & TRARALGON bus arriving. Direction: Bairnsdale BAIRNSDALE - MELBOURNE VIA SALE & 8 stops TRARALGON bus Time Schedule VIEW LINE SCHEDULE Bairnsdale Route Timetable: Sunday Not Operational Traralgon Railway Station (Traralgon) Monday 7:45 AM - 7:27 PM Latrobe St/Princes Hwy (Rosedale) Tuesday 7:45 AM - 7:27 PM 74 Prince Street, Rosedale Wednesday 7:45 AM - 7:27 PM Hunt Pl/Princes Hwy (Wurruk) Thursday 7:45 AM - 7:27 PM Gippsland Shopping Centre/Cunninghame St Friday 7:45 AM - 7:27 PM (Sale) Cunninghame Street, Sale Saturday 5:14 PM Sale Railway Station (Sale) Stratford Railway Station (Stratford) BAIRNSDALE - MELBOURNE VIA SALE & Lindenow Rd/Princes Hwy (Lindenow) TRARALGON bus Info Direction: Bairnsdale Bairnsdale Railway Station (Bairnsdale) Stops: 8 111 Macleod Street, Bairnsdale Trip Duration: 105 min Line Summary: Traralgon Railway Station (Traralgon), Latrobe St/Princes Hwy (Rosedale), Hunt Pl/Princes Hwy (Wurruk), Gippsland Shopping Centre/Cunninghame St (Sale), Sale Railway Station (Sale), Stratford Railway Station (Stratford), Lindenow Rd/Princes -
Veterinary Pathology Report
VETERINARY PATHOLOGY REPORT Australian Society for Veterinary Pathology Brought to you by: New South Wales Agriculture Elizabeth Macarthur Agriculture Institute Private Bag 8 Camden NSW 2570 Registered by Australia Post Publication No. VBG 6333 EDITOR: Gary Reddacliff Number 31 August, 1991 PAGE CONTENTS 1 EDITOR'S REPORT 2 1991 AGM MINUTES STATE REPORTS 15 Queensland (Fraser Trueman) 19 Victoria (John Mackie) 19 South Australia (Vui Ling Tham) 21 New South Wales (Paul Gill) 25 Western Australia (Ron Peet) Northern Territory (Lorna Melville) Tasmania (to be advised) 30 LETTERS TO THE EDITOR DEADLINE FOR NEXT VET. PATH. REPORT IS OCTOBER 1, 1991 1. FROM THE EDITOR Welcome to VPR issue No. 31! This issue has been a little delayed in the changeover of the executive to NSW. However, a wealth of Interesting material has been forthcoming and we hope to continue the high standard set by previous executives. Please submit material for the next issue (expected in October/November) to your state representatives as soon as possible to allow it to be produced on time. Deadline for receipt of material will be October 1. A reminder to all members - Please keep us advised of any change of address so that you will continue to receive your copy of VPR! The roles of secretary and newsletter editor are now somewhat combined and although all correspondence should still be addressed to the secretary, many of the functions will necessarily be undertaken by other members of the executive. All membership enquiries will be bandied by our capable new treasurer, Edla Arzey. Specialist registration and training matters will be the province of Tony Ross and Keith Walker, while matters concerning the Pathology Registry will be fielded by the ongoing management committee. -

News Release
The Male Bag Foundation Media Release Fighting Prostate Cancer News Release Dubbo Health Service to introduce leading prostate cancer biopsy procedure thanks to The Male Bag Foundation Key points • The Male Bag Foundation (MBF) has provided a $60,000 grant to the Dubbo Health Service (DHS) to purchase a transperineal biopsy machine (TBM). The grant is the Foundation’s equal highest for 2019. • Foundation Patron David Parkin OAM said “the Foundation is a purpose-built charity working to reduce the impact of prostate cancer in regional communities, and we welcome the opportunity to accelerate the delivery of world class prostate cancer biopsy services to such a large and diverse health district in NSW.” He went on to say, “The Foundation’s riders and support team on our 2,000 km postie bike ride last October experienced the vastness of the Western Health Care District first-hand, and our experience tells us that the convenience and improved health outcomes from TBM services will be a game-changer for men, their families and towns from Bourke to Canowindra. Being able to help people in the bush really drives us.” • Male Bag Foundation Chairman Robert Glover said “the grant acknowledges the great support we received along our 2018 ride including a number of local councils and community service clubs. We look forward to continuing our support to Western NSW.” • Scott McLachlan, Chief Executive of Western NSW Local Health District said “We would like to thank the Foundation. They appeared from no-where on their postie bikes raising funds to fight prostate cancer, and now their generosity will ensure that advanced biopsy services will be available in Dubbo in a just few months.” • The incidence of prostate cancer in regional NSW is much higher than Australia’s major cities. -

Trail Bike Riding – Bairnsdale and Surrounds FS0113 DSE – Bairnsdale ISSN
August 2010 Trail Bike Riding – Bairnsdale and surrounds FS0113 DSE – Bairnsdale ISSN Mount Taylor Trail Bike Visitor Area Safety Information Situated on the Bullumwaal Road is the Mount Taylor Forest roads and tracks Trail Bike Visitor Area. Located 15 km north of Bairnsdale, All roads and tracks, including the sign posted riding at the foot of Mount Taylor, the visitor area serves as an routes, are two-way and open to other vehicles. Be excellent launching place for your ride within the local aware of other vehicles that share the road with you. State Forest. Seasonal road closure Location and Access Some forest roads are subject to seasonal road closure. These roads are closed from the Thursday after the Bairnsdale is approximately 275km east of Melbourne, on Queens Birthday Long weekend in June until the the Princes Highway. To access the Mount Taylor Trail weekend before Melbourne Cup Day in November. For Bike Visitor Area from Bairnsdale, follow the signs to Wy more information about seasonal road closures visit Yung, and then travel north along the Bullumwaal Road www.parkweb.vic.gov.au. for approximately 10km. At the point where the bitumen turns to gravel, the Trail Bike Visitor Area can be EPIRB/PLB accessed via the gravel road on your left hand side Personal locator beacons are available for loan from the (Lookout Road). The visitor area is located another 300m Bairnsdale Police Station.43-45 Main St. Bairnsdale. Phone: (03) 5150 2600 further up this road (past the picnic area). What Facilities are Provided? Ride for Tomorrow -

SIW2019 Events
Start Date Event Title Event Type Event Description Organiser Name State 24/10/2019 Territorian Santa Fun Run Sport Join us for our 9th Annual Santa Fun Run in Darwin on Sunday 24 November 2019. Easy 2km course route around the Darwin Waterfront! NT Register online and have an exciting morning run or walk with family and friends! Funds raised will go towards the amazing Variety NT Starfish Swim Group, a free one-on-one swim group for children with disability, ages from 2 to 18 years. Mums and Bubs classes also available. Timeline of Events on the day: 6:00-7:00am – Pick-up your Santa suit (in front of the Convention Centre) * 7:15am – Jolly Zumba warm-up 7:30am – Santa Fun Run start 8:15am – Presentations 8:20am – Lucky Bib prize draw ** 8:30am – Santa meet & greet Other Activities: CGee face-painting Giant slip ‘n Slide DJ Kev Fundraising Sausage Sizzle and raffle 15/11/2019 GIPPSLAND GROOVERS CLUB Other The Gippsland Groovers Club Christmas Party is on the horizon! Have a dance and meal together as part of social inclusion week. Gippsland Groovers Club VIC CHRISTMAS PARTY There will be a live band and lucky door prizes, plus a special first door prize. People who require support or supervision must be accompanied by a carer who must have a pre-purchased dinner ticket. Tickets are required and can be collected from Mawarra Centre in Warragul, Moe Life Skills, Moe Podicare Shoe Shop 20/11/2019 Bairnsdale Squash and Table Tennis Sport Bairnsdale Squash and Table Tennis Centre in celebration of Social Inclusion Week and international Day of People with Disability, welcomes the Bairnsdale Squash and VIC Centre - All Abilities Open Day community to their All Abilities Open Day. -
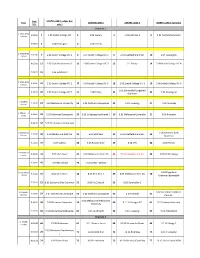
2019 Head of the Yarra Draft Start Order V5.Xlsx
Start SOUTH LANE ( judges box Event CENTRE LANE 1 CENTRE LANE 2 NORTH LANE ( Far Side ) time side ) Bracket 1 1. MSCH8+O1 9:00:00 1 6 crews 1.05 Scotch College VIC 2 1.06 Sydney 3 1.03 Hutchins 1 4 1.01 Caulfield Grammar 9:00:50 5 1.04 Newington 6 1.02 Friends 2. MSCH8+O2 9:05:00 7 9 crews 2.06 Scotch College VIC 1 8 2.07 Scotch College VIC 2 9 2.01 Caulfield Grammar 10 2.04 Newington 9:05:40 11 2.02 Caulfield Grammar 2 12 2.08 Scotch College VIC 3 13 2.1 Trinity 14 2.09 Scotch College VIC 4 9:06:20 15 2.03 Hutchins 2 3. MSCH8+O3 9:10:00 16 8 crews 3.03 Scotch College VIC 1 17 3.04 Scotch College VIC 2 18 3.05 Scotch College VIC 3 19 3.06 Scotch College VIC 4 3.01 Bairnsdale/Gippsland 9:10:40 20 3.07 Scotch College VIC 5 21 3.08 Trinity 22 23 3.02 Newington Grammar 4. MMH8+ 9:15:00 24 4 crews 4.02 Melbourne University 25 4.03 North Esk Composite 26 4.04 Toowong 27 4.01 Corio Bay 5. MMI8+ 5 9:18:00 28 crews 5.03 Richmond Composite 29 5.05 St George/Leichhardt 1 30 5.02 Melbourne University 31 5.04 Riverside 9:18:40 32 5.01 Drummoyne Composite 6. FSCH8+O1 6.01 Canberra Girls 9:23:00 33 6.09 Melbourne Girls' GS 34 6.04 MLC Kew 35 6.02 Caulfield Grammar 36 8 crews Grammar 9:23:40 37 6.07 Sydney 38 6.06 Ruyton Girls' 39 6.08 UTS 40 6.03 Friends 7.