Lepidoptera, Pyralidae) from China
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Mitochondrial Genome of Ephestia Elutella (Insecta: Lepidoptera: Pyralidae)
MITOCHONDRIAL DNA PART B: RESOURCES, 2018 VOL. 3, NO. 1, 189–190 https://doi.org/10.1080/23802359.2018.1436993 MITOGENOME ANNOUNCEMENT The mitochondrial genome of Ephestia elutella (Insecta: Lepidoptera: Pyralidae) Qiongyou Liua, Xiaohong Jianga, Xiaohui Houa, Hong Yangb and Wenlong Chenc,d aDepartment of Basic Medical Sciences, Zunyi Medical University, Zunyi, Guizhou, P. R. China; bInstitute of Entomology, College of Tobacco Science, Guizhou University, Guiyang, Guizhou, P. R. China; cGuizhou Provincial Key Laboratory for Agricultural Pest Management of Mountainous Region, Guiyang, Guizhou, P R China; dSpecial Key Laboratory for Development and Utilization of Insect Resources of Guizhou, Institute of Entomology, Guizhou University, Guiyang, Guizhou, P. R. China ABSTRACT ARTICLE HISTORY In this study, the complete mitochondrial genome of the tobacco moth Ephestia elutella was sequenced Received 9 January 2018 and analyzed. The mitochondrial genome is 15345 bp long and contains 13 protein-coding genes, two Accepted 18 January 2018 rRNA genes, 22 tRNA genes, and one control region. Twenty-three genes were found to be encoded by KEYWORDS the majority strand and the other 14 genes by minority strand, those is similar to that of other insects. Mitochondrial genome; The nucleotide compositing of the majority strand are 38.6% of A, 11.77% of C, 42.05% of T and 7.58% Pyralidae; phylogenetic; of G. The phylogenetic analysis by Maximum-likelihood (ML) method revealed that the E. elutella was Ephestia elutella close to the same genus insect Ephestia kuehniella. The tobacco moth Ephestia elutella (Hubner)€ (Lepidoptera: Mitogenomics Consortium 2017; Singh et al. 2017). Overall Pyralidae) is one of the main pests in stored tobacco, and it nucleotide compositions of the majority strand are 38.6% of is cosmopolitan in distribution (Ashworth 1993). -

Family GEOMETRID^
OF WASHINGTON. 29 followed and the only one which does justice to the authors of subsequently-erected genera. The following paper by Dr. Dyar was read by title : NEW NORTH AMERICAN LEPIDOPTERA AND SYNONYM- ICAL NOTES. BY HARRISON G. DYAR. The following paper is in large part the result of a study of a collection of some 1,500 specimens of Phycitinae which Mr. W. D. Kearfott has placed at my disposal. Family NOCTUID^. Taeniocampa terminatissima Dyar. l This species proves to be synonymous with Trichorthosia parallela Grote, as Mr. Jacob Doll has kindly pointed out to me. The species is included in Hampson's fourth volume of the Catalogue of Lepidoptera Phalaenae, but the genus is not in the table. The spined hind tibiae and hairy eyes make the form characteristic. Family GEOMETRID^. Tephroclystia harlequinaria, new species. with Fore wings light stone-gray patches of light ocherous. The largest patch occupies the space between veins 3 and 4, overspreading these veins a little and running to the fringe; there is a small patch at the extreme base of wing, a diffuse, indistinctly doubled one on costa at t.-p. line and a small one on vein 6 to outer margin. Lines blackish, waved, scarcely cutting the ocherous shades, marked by little black dashes on the median vein and on all the veins in the t.-p. line, which is regularly and evenly bent outward. Subterminal line fine, white, scarcely enlarged above anal angle. Discal spot black. Hind wings whitish on costal half, the lines distinct near inner margin. A whitish patch at base of abdomen dorsally. -
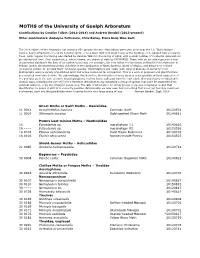
Moth Records
MOTHS of the University of Guelph Arboretum Contributions by Candice Talbot (2012-2014) and Andrew Bendall (2013-present) Other contributors: Andalyne Tofflemire, Chris Earley, Fiona Reid, Mike Kent The 2018 edition of the Arboretum list contains 851 species of moth. Most adults were seen at or near the J.C. Taylor Nature Centre, being attracted to the white building lights, or to a black light and sheet hung by the building, or to painted bait on nearby trees. Semi-regular monitoring was started by Candice Talbot in the spring of 2012, with a small number of incidental observations pre-dating that time. First record dates, where known, are shown at right as YYYYMMDD. Those with an asterisk represent a first documented sighting if the date of an earlier record was not available. We now follow the taxonomy outlined in Pohl, Patterson & Pelham (2016) Annotated taxonomic checklist of the Lepidoptera of North America, North of Mexico, and adopt their revised numbering system for all valid North American species. Identifications are made (with varying degrees of certainty) from photographs using a variety of published print and online resources for comparison. This is a work in progress and identifications are revisited from time to time. We acknowledge that definitive identification of some species is not possible without dissection of the genitalia or, in the case of some microlepidoptera, rearing larvae collected from the host plant. Our uncertainty is indicated in various ways, including the use of [t] for a tentative identification, by indicating a group of species that can't be separated from external features, or by identifying to genus only. -

Predator to Prey to Poop: Bats As Microbial Hosts and Insectivorous Hunters
Predator to Prey to Poop: Bats as Microbial Hosts and Insectivorous Hunters A Thesis SUBMITTED TO THE FACULTY OF THE UNIVERSITY OF MINNESOTA BY Miranda Galey IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE Dr. Ron Moen, Dr. Jessica R. Sieber September 2020 Copyright © Miranda Galey 2020 Abstract Bat fecal samples are a rich source of ecological data for bat biologists, entomologists, and microbiologists. Feces collected from individual bats can be used to profile the gut microbiome using microbial DNA and to understand bat foraging strategies using arthropod DNA. We used eDNA collected from bat fecal samples to better understand bats as predators in the context of their unique gut physiology. We used high through- put sequencing of the COI gene and 16S rRNA gene to determine the diet composition and gut microbiome composition of three bat species in Minnesota: Eptesicus fuscus, Myotis lucifugus and M. septentrionalis. In our analysis of insect prey, we found that E. fuscus consistently foraged for a higher diversity of beetle species compared to other insects. We found that the proportional frequency of tympanate samples from M. septentrionalis and M. lucifugus was similar, while M. septentrionalis consistently preyed more often upon non-flying species. We used the same set of COI sequences to determine presence of pest species, rare species, and insects not previously observed in Minnesota. We were able to combine precise arthropod identification and the for- aging areas of individually sampled bats to observe possible range expansion of some insects. The taxonomic composition of the bat gut microbiome in all three species was found to be consistent with the composition of a mammalian small intestine. -

Order Family Subfamily Genus Species Subspecies Author Year Series Region Units Lepidoptera Crambidae Acentropinae Acentria Ephe
Order Family Subfamily Genus species subspecies author year series region units Lepidoptera Crambidae Acentropinae Acentria ephemerella (Denis & Schiffermüller) 1C, 1D Nearctic, Palearctic trays Lepidoptera Crambidae Acentropinae Anydraula glycerialis (Walker) 1D Australasian trays Lepidoptera Crambidae Acentropinae Argyractis berthalis (Schaus) 1C Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis dodalis Schaus 1B Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis elphegalis (Schaus) 1B Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis flavalis (Warren) 1B Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis iasusalis (Walker) 1D Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis paulalis (Schaus) 1D Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis sp. 1C, 1D Neotropical trays Lepidoptera Crambidae Acentropinae Argyractis tetropalis Hampson 1D African trays Lepidoptera Crambidae Acentropinae Argyractis triopalis Hampson 1D African trays Lepidoptera Crambidae Acentropinae Argyractoides catenalis (Guenée 1D Neotropical trays Lepidoptera Crambidae Acentropinae Argyractoides chalcistis (Dognin) 1D Neotropical trays Lepidoptera Crambidae Acentropinae Argyractoides gontranalis (Schaus) 1D Neotropical trays Lepidoptera Crambidae Acentropinae Aulacodes acroperalis Hampson 1D Australasian trays Lepidoptera Crambidae Acentropinae Aulacodes adiantealis (Walker) 1D Neotropical trays Lepidoptera Crambidae Acentropinae Aulacodes aechmialis Guenée 1D Neotropical trays Lepidoptera -

And Phylogenetic Analysis of Pyraloidea
insects Article The First Mitogenomes of the Subfamily Odontiinae (Lepidoptera, Crambidae) and Phylogenetic Analysis of Pyraloidea Mujie Qi 1, Huifeng Zhao 2,3 , Fang Yu 4, Aibing Zhang 3 and Houhun Li 1,* 1 College of Life Sciences, Nankai University, Tianjin 300071, China; [email protected] 2 Hebei Key Laboratory of Animal Diversity, College of Life Science, Langfang Normal University, Langfang 065000, China; [email protected] 3 College of Life Sciences, Capital Normal University, Beijing 100048, China; [email protected] 4 Jiangsu Key Laboratory of Biofunctional Molecule, School of Life Sciences, Chemistry & Chemical Engineering, Jiangsu Second Normal University, Nanjing 211200, China; [email protected] * Correspondence: [email protected] Simple Summary: The Odontiinae is a small group in the Pyraloidea comprised of 388 species in 88 genera, but externally, these moths are diverse, including heterogeneous maculation and a size range from 9 to 50 mm in total wingspan. The monophyly of Pyraloidea and the two families (Pyralidae and Crambidae) is well supported by phylogenetic analyses based on morphology and molecular data of multiple nuclear genes. However, only a few mito-phylogenetic analyses have been conducted and no mitogenome of Odontiinae species has been reported. Three complete mitogenomes of odontiine species were sequenced and analyzed for the first time herein. The results showed that Odontiinae mitogenomes shared similar genomic characters with other Pyraloidea. The phylogenetic analyses based on 13 PCGs of mitogenomes confirmed the monophyly of Odontiinae and its position Citation: Qi, M.; Zhao, H.; Yu, F.; within Crambidae. Zhang, A.; Li, H. The First Mitogenomes of the Subfamily Abstract: The complete mitochondrial genomes of three species of Odontiinae were newly sequenced: Odontiinae (Lepidoptera, Crambidae) Dausara latiterminalis Yoshiyasu, Heortia vitessoides (Moore), and Pseudonoorda nigropunctalis (Hamp- and Phylogenetic Analysis of son). -

Taxa List: Invertebrates
Taxa List: Current as of Invertebrates 2019-Feb-13 For an explanation of Subnational(S), National(N) and Global(G) Ranks, refer to: www.biodiversity.sk.ca/ranking.htm ID: Scientific Name: Common Name: G-Rank: N-Rank: S-Rank: COSEWIC: Arachnida - Arachnids Araneae - Spiders Agelenidae 9967 Agelenopsis actuosa Funnel Weaver Spider GNR N4N5 S4 A Grass Spider 9968 Agelenopsis oklahoma A Funnel Spider G5 N5 S4 A Grass Spider 27381 Agelenopsis potteri A Grass Spider G5 N5 S4 9969 Agelenopsis utahana A Grass Spider G5 N5 S5 403295 Eratigena atrica Giant House Spider GNR NNA SNA Tegenaria atrica Tegenaria gigantea Tegenaria duellica 27150 Tegenaria domestica Barn Funnel Weaver GNR NNA SNA Amaurobiidae 27382 Amaurobius borealis a hackledmesh weaver G5 N5 S4 10011 Arctobius agelenoides Common Amaurobiid Spider G5 N5 S4 10016 Callioplus euoplus Debris Amaurobiid Spider G5 N5 S4 10003 Callobius nomeus A Hackledmesh Weaver G5 N5 S4 Anyphaenidae 10007 Anyphaena pacifica A Ghost Spider G5 N5 SU Araneidae - Orbweavers 10038 Aculepeira packardii An Orbweaver Spider G5 N5 S4 Aculepeira packardi 10002 Araneus corticarius An Orbweaver Spider G5 N5 S4 9999 Araneus gemmoides An Orbweaver Spider G5 N5 S4 27479 Araneus iviei An Orbweaver Spider G5 N5 S4 27100 Araneus marmoreus Marbled Orbweaver G5 N5 S5 27480 Araneus nordmanni An Orbweaver Spider G5 N5 S4 10000 Araneus saevus An Orbweaver Spider G5 N5 S5 10001 Araneus trifolium Shamrock Orbweaver Spider G5 N5 S5 10009 Araniella displicata Six-spotted Orb Weaver G5 N5 S5 10010 Araniella proxima An Orbweaver Spider -

Craters of the Moon
National Monument and Preserve U.S. Department of the Interior 1 Craters of the Moon o Butterflies and Moths (Lepidoptera) of CRMO Butterflies and moths are some of the most widely-distributed and recognizable insects. They are found worldwide in nearly every habitat, comprising an estimated 174,250 species. About 700 butterflies and 11,000 moths are found in North America. Over 300 species of Lepidoptera have been documented on Craters of the Moon (CRMO) another 250 may occur here. Edith’s Checkerspot Lepidopterans feed primarily on nectar from flowers but may derive nourishment from pollen, tree sap, rotting fruit, dung or decaying flesh, and they can get dissolved minerals from wet sand or dirt. Many, however, do not feed at all as adults. They are important as pollinators and are capable of moving pollen over great distances. Lepidopterans have a four stage life cycle consisting of the egg, larva (caterpillar) and chrysalis (cocoon) before finally emerging as fully formed adults. They employ just about every strategy to avoid winter. Some migrate long distances, others hibernate and some completely die off leaving only the eggs to survive winter and begin the next generation. Weidemeyer's Admiral This list is a work in progress, with so many species in this insect order, it likely always will be! Your observations, pictures and collected specimens (already dead only please) are welcomed and encouraged so that we may continue to build our knowledge of these diverse species. Melissa Blue All species on this list have been either documented on CRMO, in one (or more) of the 5 counties CRMO overlaps or elsewhere on the Snake River Plain. -

Moths and Butterflies of Vermont (Lepidoptera): a Faunal Checklist
~ . I CO". ..... , . ~ 'DL5?lA'1 CoP'! Moths-and Butterflies of Vermont (Lepidoptera) A Faunal Checklist Agricultural Experiment Station, University of Vermont Department of Forests, Parks and Recreation, State of Vermont Miscellaneous Publication 116 Vermont l\I1onitoring Cooperative Bulletin No.1 January 1995 iii A KN Wl DGMENTS Moths and Butter-flies of Vermont (Lepidoptera) I ntcmolocv Research Laboratory collection by J. Boone and J. r , I. A. Prll ,I . N. n , . Curtis, P. M. Hanson, and B. Heinrich. Collections from Jericho w ll h III C op r tlon of D. R. Tobi who maintained light trap operations during ull f r to the Zadock Thompson Zoological Collections (UVMl, and R. A Faunal Checklist , t it m torlat. J. luzatto and D. Dillner kindly brought the Sphinx eremitus Sp II t IItll l tl II W ro kindly provided by; K. B. Bolte, P. T. Dang, J. D. Lafontaine, B. Landry, J.-F. landry, nd A . Mu illur l ntr tor I mel nnd Biological Resources Research, Agriculture and Agrifood, Canada); R. L. Brown 1M I Iplll l il t III II I Mu um); D. Adamski, J . Burns, D. R. Davis, D. C. Ferguson, R. W. Hodn , M.J' I' Ill , 11111 1. 1( , II I) In lSmlthsonian Institution); T. L. McCabe lNew York State Museum); W. Millo r lUnl v r ltv of Mill" I ); .. Oumter, and F. H. Rindge (American Museum of Natural History); J. E. Rawl/nll ( nu 01 Mil 11111 , N tur I til! tory); D. F. Schweitzer (The Nature Conservancy); J. G. Franclemont lCornflll Unlv r tV); I I. -

An All-Taxa Biodiversity Inventory of the Huron Mountain Club
AN ALL-TAXA BIODIVERSITY INVENTORY OF THE HURON MOUNTAIN CLUB Vers io n: February 2020 Cite as: Woods, K.D. (Compiler). 2020. An all-taxa biodiversity inventory of the Huron Mountain Club. Version February 2020. Occasional papers of the Huron Mountain Wildlife Foundation, No. 5. [http://www.hmwf.org/species_list.php] Introduction and general compilation by: Kerry D. Woods Natural Sciences Bennington College Bennington VT 05201 Kingdom Fungi compiled by: Dana L. Richter School of Forest Resources and Environmental Science Michigan Technological University Houghton, MI 49931 DEDICATION This project is dedicated to Dr. William R. Manierre, who is responsible, directly and indirectly, for documenting a large proportion of the taxa listed here. INTRODUCTION No complete species inventory exists for any area. Particularly charismatic groups – birds, large mammals, butterflies – are thoroughly documented for many areas (including the Huron Mountains), but even these groups present some surprises when larger or more remote areas are examined closely, and range changes lead to additions and subtractions. Other higher-level taxa are generally much more poorly documented; even approximate inventories exist for only a few, typically restricted locales. The most diverse taxa (most notably, in terrestrial ecosystems, insects) and many of the most ecologically important groups (decay fungi, soil invertebrates) are, with few exceptions, embarrassingly poorly documented. The notion of an ‘all-taxon biodiversity inventory’ (or ATBI) – a complete listing of species, of all taxonomic groups for a defined locale – is of relatively recent vintage, originating with ecologist Daniel Janzen’s initiative to fully document the biota of Costa Rica’s Guanacaste National Park. Miller (2005) offers a brief a history of ATBI efforts, and notes that only three significant regional efforts appear to be ongoing. -

Table of Contents Insect Happening In
=========================================================================================== Vol. 21, No. 2 May, 2017 =========================================================================================== convened between the Park and involved partners to review the blitzes and consider a path forward. The Park felt there was not enough value to be gained by continuing blitzes in their current form – although as you can see from the report in this issue on the 2016 blitz that significant numbers of new finds continued to be made. The park also did not have the resources to subsidize the blitz, nor were they interested in pursuing grants for support. The partners felt that participants were not willing to pay hundreds of dollars to share their expertise. So the blitzes at Acadia National Park have come to an end. We parted on amicable terms and the Park is still interested in having Entomological projects happen at ANP. Various ideas were considered, but nothing is in the works at this time. Logistics is the biggest problem; there is no place to stay near MDI for a reasonable cost, traffic is heavy and it's far enough away for most of us that travelling back and forth is not an option. A couple projects that were discussed were working on the insect portion of the collection or processing by-catch from a research project if one comes up – these are things that could be done in the off-season. Plan on heading to the new Katahdin Woods and Waters People are asking “What is happening with the Bioblitz Monument August 19th though, and help find what lives at Acadia?” there. Prices are more reasonable and it is a beautiful part of The Maine Entomological Society has partnered with Maine. -

Characterization of the Complete Mitochondrial Genome of Orthaga Olivacea Warre (Lepidoptera Pyralidae) and Comparison with Other Lepidopteran Insects
PLOS ONE RESEARCH ARTICLE Characterization of the complete mitochondrial genome of Orthaga olivacea Warre (Lepidoptera Pyralidae) and comparison with other Lepidopteran insects Liangli Yang1☯, Junjun Dai2☯, Qiuping Gao1, Guozhen Yuan1, Jiang Liu1, Yu Sun1, 1 1 1 1 1 1 Yuxuan Sun , Lei Wang , Cen Qian , Baojian Zhu , Chaoliang Liu , Guoqing WeiID * a1111111111 1 School of Life Sciences, Anhui Agricultural University, Hefei, P. R. China, 2 Sericultural Research Institute, Anhui Academy of Agricultural Sciences, Hefei, P. R. China a1111111111 a1111111111 ☯ These authors contributed equally to this work. a1111111111 * [email protected] a1111111111 Abstract Orthaga olivacea Warre (Lepidoptera: Pyralidae) is an important agricultural pest of cam- OPEN ACCESS phor trees (Cinnamomum camphora). To further supplement the known genome-level fea- Citation: Yang L, Dai J, Gao Q, Yuan G, Liu J, Sun tures of related species, the complete mitochondrial genome of Orthaga olivacea is Y, et al. (2020) Characterization of the complete mitochondrial genome of Orthaga olivacea Warre amplified, sequenced, annotated, analyzed, and compared with 58 other species of Lepi- (Lepidoptera Pyralidae) and comparison with other dopteran. The complete sequence is 15,174 bp, containing 13 protein-coding genes Lepidopteran insects. PLoS ONE 15(3): e0227831. (PCGs), 22 transfer RNA (tRNA) genes, 2 ribosomal RNA (rRNA) genes, and a putative https://doi.org/10.1371/journal.pone.0227831 control region. Base composition is biased toward adenine and thymine (79.02% A+T) and Editor: Jeffrey M. Marcus, University of Manitoba, A+T skew are slightly negative. Twelve of the 13 PCGs use typical ATN start codons. The CANADA exception is cytochrome oxidase 1 (cox1) that utilizes a CGA initiation codon.