The Stirling Engine: the Heat Engine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abstract 1. Introduction 2. Robert Stirling
Stirling Stuff Dr John S. Reid, Department of Physics, Meston Building, University of Aberdeen, Aberdeen AB12 3UE, Scotland Abstract Robert Stirling’s patent for what was essentially a new type of engine to create work from heat was submitted in 1816. Its reception was underwhelming and although the idea was sporadically developed, it was eclipsed by the steam engine and, later, the internal combustion engine. Today, though, the environmentally favourable credentials of the Stirling engine principles are driving a resurgence of interest, with modern designs using modern materials. These themes are woven through a historically based narrative that introduces Robert Stirling and his background, a description of his patent and the principles behind his engine, and discusses the now popular model Stirling engines readily available. These topical models, or alternatives made ‘in house’, form a good platform for investigating some of the thermodynamics governing the performance of engines in general. ---------------------------------------------------------------------------------------------------------------- 1. Introduction 2016 marks the bicentenary of the submission of Robert Stirling’s patent that described heat exchangers and the technology of the Stirling engine. James Watt was still alive in 1816 and his steam engine was gaining a foothold in mines, in mills, in a few goods railways and even in pioneering ‘steamers’. Who needed another new engine from another Scot? The Stirling engine is a markedly different machine from either the earlier steam engine or the later internal combustion engine. For reasons to be explained, after a comparatively obscure two centuries the Stirling engine is attracting new interest, for it has environmentally friendly credentials for an engine. This tribute introduces the man, his patent, the engine and how it is realised in example models readily available on the internet. -

Feasibility Study Into Stirling Engines Application in Ship's Energy Systems
Transactions on the Built Environment vol 42, © 1999 WIT Press, www.witpress.com, ISSN 1743-3509 Feasibility study into Stirling engines application in ship's energy systems S. Zmudzki, Faculty of Maritime Technology, Technical University of Szczecin, 71- 065 Szczecin, al Piastow 41, Poland E-mail: szmudzkia@shiptech. tuniv.szczecin.pl Abstract The possibility of utilization of solar energy as well as exhaust gas energy from main and auxiliary marine diesel engines for driving Stirling engines has been analysed. The Stirling engines are used for driving current generators. The solar dish Stirling units of 100 kW total effective power may be installed on a board of different type of ships. The system utilizing the exhaust gas energy has been provided with three-way catalyst which may lead to the reduction of emissions below limits established by appropriate regulations. 1 Introduction The Stirling engine belongs to a group of piston engines with so called external supply of heat energy, flowing then through metal walls of engine heater into working gas circulating in the working space. The heat energy supply may be realized or by means of direct effect of the high temperature energy source or by an intermediate heating system i.e. heat pipe or pool-boiler receiver. Thus, the design provides for the possibility of application of different types of heat energy of sufficiently large power. In particular, the advantages of Stirling engines are set off when using the alternative energy sources, especially waste heat energy, the operating costs for which are relatively lower in comparison with capital costs. In case of ship s energy installations, the solar energy available at appropriate geographical latitudes for ships having very large open deck surface / i.e. -

Novel Hot Air Engine and Its Mathematical Model – Experimental Measurements and Numerical Analysis
POLLACK PERIODICA An International Journal for Engineering and Information Sciences DOI: 10.1556/606.2019.14.1.5 Vol. 14, No. 1, pp. 47–58 (2019) www.akademiai.com NOVEL HOT AIR ENGINE AND ITS MATHEMATICAL MODEL – EXPERIMENTAL MEASUREMENTS AND NUMERICAL ANALYSIS 1 Gyula KRAMER, 2 Gabor SZEPESI *, 3 Zoltán SIMÉNFALVI 1,2,3 Department of Chemical Machinery, Institute of Energy and Chemical Machinery University of Miskolc, Miskolc-Egyetemváros 3515, Hungary e-mail: [email protected], [email protected], [email protected] Received 11 December 2017; accepted 25 June 2018 Abstract: In the relevant literature there are many types of heat engines. One of those is the group of the so called hot air engines. This paper introduces their world, also introduces the new kind of machine that was developed and built at Department of Chemical Machinery, Institute of Energy and Chemical Machinery, University of Miskolc. Emphasizing the novelty of construction and the working principle are explained. Also the mathematical model of this new engine was prepared and compared to the real model of engine. Keywords: Hot, Air, Engine, Mathematical model 1. Introduction There are three types of volumetric heat engines: the internal combustion engines; steam engines; and hot air engines. The first one is well known, because it is on zenith nowadays. The steam machines are also well known, because their time has just passed, even the elder ones could see those in use. But the hot air engines are forgotten. Our aim is to consider that one. The history of hot air engines is 200 years old. -

25 Kw Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications
25 kW Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications Lee SMITHa, Brian NUELa, Samuel P WEAVERa,*, Stefan BERKOWERa, Samuel C WEAVERb, Bill GROSSc aCool Energy, Inc, 5541 Central Avenue, Boulder CO 80301 bProton Power, Inc, 487 Sam Rayburn Parkway, Lenoir City TN 37771 cIdealab, 130 W. Union St, Pasadena CA 91103 *Corresponding author: [email protected] Keywords: Stirling engine, waste heat recovery, concentrating solar power, biomass power generation, low-temperature power generation, distributed generation ABSTRACT This paper covers the design, performance optimization, build, and test of a 25 kW Stirling engine that has demonstrated > 60% of the Carnot limit for thermal to electrical conversion efficiency at test conditions of 329 °C hot side temperature and 19 °C rejection temperature. These results were enabled by an engine design and construction that has minimal pressure drop in the gas flow path, thermal conduction losses that are limited by design, and which employs a novel rotary drive mechanism. Features of this engine design include high-surface- area heat exchangers, nitrogen as the working fluid, a single-acting alpha configuration, and a design target for operation between 150 °C and 400 °C. 1 1. INTRODUCTION Since 2006, Cool Energy, Inc. (CEI) has designed, fabricated, and tested five generations of low-temperature (150 °C to 400 °C) Stirling engines that drive internally integrated electric alternators. The fifth generation of engine built by Cool Energy is rated at 25 kW of electrical power output, and is trade-named the ThermoHeart® Engine. Sources of low-to-medium temperature thermal energy, such as internal combustion engine exhaust, industrial waste heat, flared gas, and small-scale solar heat, have relatively few methods available for conversion into more valuable electrical energy, and the thermal energy is usually wasted. -

A Stirling Engine for Automotive Applications Steve Djetel, Sylvie Bégot, François Lanzetta, Eric Gavignet, Wissam S
A Stirling Engine for Automotive Applications Steve Djetel, Sylvie Bégot, François Lanzetta, Eric Gavignet, Wissam S. Bou Nader To cite this version: Steve Djetel, Sylvie Bégot, François Lanzetta, Eric Gavignet, Wissam S. Bou Nader. A Stirling Engine for Automotive Applications. Vehicle Power and Propulsion Conference, Dec 2017, Belfort, France. hal-02131002 HAL Id: hal-02131002 https://hal.archives-ouvertes.fr/hal-02131002 Submitted on 16 May 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. A Stirling engine for automotive applications Steve Djetel, Sylvie Bégot, François Lanzetta, Wissam S. Bou Nader Eric Gavignet Technical center of Vélizy FEMTO-ST Institute PSA Group Univ. Bourgogne Franche-Comté, CNRS 78943 Velizy, France Département ENERGIE [email protected] 90000 Belfort, France [email protected] Abstract — This paper describes a Stirling Engine used as an the fuel and air are combusted under pressure and expanded Auxiliary Power Unit in a Series Hybrid Vehicle. The engine directly to produce work [4-5]. The Stirling technology is modeling is presented, then its integration in the vehicle power interesting for two major raisons: (1) the theoretical train is described. -

Comparison of ORC Turbine and Stirling Engine to Produce Electricity from Gasified Poultry Waste
Sustainability 2014, 6, 5714-5729; doi:10.3390/su6095714 OPEN ACCESS sustainability ISSN 2071-1050 www.mdpi.com/journal/sustainability Article Comparison of ORC Turbine and Stirling Engine to Produce Electricity from Gasified Poultry Waste Franco Cotana 1,†, Antonio Messineo 2,†, Alessandro Petrozzi 1,†,*, Valentina Coccia 1, Gianluca Cavalaglio 1 and Andrea Aquino 1 1 CRB, Centro di Ricerca sulle Biomasse, Via Duranti sn, 06125 Perugia, Italy; E-Mails: [email protected] (F.C.); [email protected] (V.C.); [email protected] (G.C.); [email protected] (A.A.) 2 Università degli Studi di Enna “Kore” Cittadella Universitaria, 94100 Enna, Italy; E-Mail: [email protected] † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +39-075-585-3806; Fax: +39-075-515-3321. Received: 25 June 2014; in revised form: 5 August 2014 / Accepted: 12 August 2014 / Published: 28 August 2014 Abstract: The Biomass Research Centre, section of CIRIAF, has recently developed a biomass boiler (300 kW thermal powered), fed by the poultry manure collected in a nearby livestock. All the thermal requirements of the livestock will be covered by the heat produced by gas combustion in the gasifier boiler. Within the activities carried out by the research project ENERPOLL (Energy Valorization of Poultry Manure in a Thermal Power Plant), funded by the Italian Ministry of Agriculture and Forestry, this paper aims at studying an upgrade version of the existing thermal plant, investigating and analyzing the possible applications for electricity production recovering the exceeding thermal energy. A comparison of Organic Rankine Cycle turbines and Stirling engines, to produce electricity from gasified poultry waste, is proposed, evaluating technical and economic parameters, considering actual incentives on renewable produced electricity. -

And High Temperature Differential Stirling Engines
COMPARISON OF LOW- AND HIGH TEMPERATURE DIFFERENTIAL STIRLING ENGINES S. IWAMOTO*1, F. TODA*1, K. HIRATA*1 M. TAKEUCHI*2 and T. YAMAMOTO*3 *1Department of Mechanical Engineering, Faculty of Engineering, Saitama University, Japan *2Suction Gas Engine MFG. Co., Ltd., Japan *3National Institute for Research and Environment, Japan ABSTRACT Stirling engines can be operated with various heat sources. We have developed several Stirling engines, included a low temperature differential Stirling engine using hot spring heat and a high temperature differential Stirling engine using a combustion gas as the fuel. These engines have different features and characteristics, and have different thermal performance. In this paper, the performances of these difference types of Stirling engines were measured, were compared using the normalized values. INTRODUCTION In the field of the low temperature differential Stirling engines, several small Stirling engines have developed by I. Kolin, J. R. Senft and so on1),2). We have being developed the low temperature differential Stirling engine with several hundreds wattage of an output power3). The engine uses hot spring heat or hot water from industrial waste heat as the fuel. In this paper, we measured heat losses and powers of the engine in order to estimate the engine performance. Then the performance of a prototype of the low temperature differential Stirling engine (LDSE) was compared with that of a high temperature differential Stirling engine (HDSE) after their experimental results had been normalized. Figure 1 Feature of LDSE STRUCTURE OF LDSE Figure 1 and Fig. 2 show the features and structure of LDSE. Table 1 shows specifications of this engine and HDSE5) used to compare the performances. -

Pursuit of Power NASA’S Propulsion Systems Laboratory No
Pursuit of Power NASA’s Propulsion Systems Laboratory No. 1 and 2 By Robert S. Arrighi National Aeronautics and Space Administration NASA History Program Office Public Outreach Division NASA Headquarters Washington, DC 20546 SP–2012–4548 2012 Library of Congress Cataloging-in-Publication Data Arrighi, Robert S., 1969- Pursuit of Power : NASA’s Propulsion Systems Laboratory, No. 1 & 2 / Robert S. Arrighi. p. cm. -- (NASA history series) (NASA SP ; 2012-4548) Includes bibliographical references and index. 1. Propulsion Systems Laboratory (U.S.)--History. 2. Wind tunnels. 3. Hypersonic wind tunnels. 4. Aerodynamics--Research--United States--History--20th century. 5. Propulsion systems--United States--History--20th century. 6. NASA Glenn Research Center. I. United States. NASA History Division. II. Title. III. Title: NASA’s Propulsion Systems Laboratory, No. 1 & 2. IV. Title: Propulsion Systems Laboratory, No. 1 & 2. TL568.P75A77 2012 629.1’1072--dc23 2011032380 • Table of Contents • Introduction ................................................................................................................................................ vii Condensed History of the PSL ......................................................................................................... ix Preserving the PSL Legacy ................................................................................................................. ix Endnotes for Introduction ................................................................................................................. -

The Gamma Stirling Engine
The Gamma Stirling Engine Leon van Dommelen July 25, 2019 Consider the Stirling cycle as shown in figure 1. Read the legend of the figure to identify the key components. The crucial stage for getting power out is (b). Here the power piston is pushing against the flywheel, increasing its speed. This part of the cycle is called the power stroke; it makes the engine produce its power. Note that most of the air in the displacer cylinder is red hot. That makes it want to expand, raising the pressure way up. (Since the air can easily move around the displacer piston, the cold air is at the same high pressure as the hot one. OK, if you insist, there is some resistance in flowing around the displacer through the regenerator stuff. But that should be small. Sometimes students mistakingly think that the hot air is at high pressure and the cold air at low. But that is not possible because there is no physical barrier between the two to support such a pressure difference. At any given stage in the cycle, it is only the density and not the pressure that is different for the hot and cold air.) The high pressure pushes forcefully against the power piston, and so on the attached flywheel, increasing the flywheel’s velocity and kinetic energy. So the device is producing power. The bad news is the later stage (d). Here the piston is going the other way. Now the flywheel must compress the air again, which slows it down. This stage is called the compression stroke. -

Stirling Engine)
Heat Cycles Hot air engine (Stirling engine) OPERATE A FUNCTIONAL MODEL OF A STIRLING ENGINE AS A HEAT ENGINE Operate the hot-air engine as a heat engine Demonstrate how thermal energy is converted into mechanical energy Measure the no-load speed as a function of the thermal power UE2060100 04/16 JS BASIC PRINCIPLES The thermodynamic cycle of the Stirling engine While the working piston is in its top dead centre posi- (invented by Rev. R. Stirling in 1816) can be pre- tion: the displacement piston retracts and air is dis- sented in a simplified manner as the processes placed towards the top end of the large cylinder so that thermal input, expansion, thermal output and com- it cools. pression. These processes have been illustrated by The cooled air is compressed by the working piston schematic diagrams (Fig. 1 to Fig. 4) for the func- extending. The mechanical work required for this is tional model used in the experiment. provided by the flywheel rod. A displacement piston P1 moves upwards and displac- If the Stirling engine is operated without any mechanical es the air downwards into the heated area of the large load, it operates with at a speed which is limited only by cylinder, thereby facilitating the input of air. During this internal friction and which depends on the input heating operation the working piston is at its bottom dead centre energy. The speed is reduced as soon as a load takes position since the displacement piston is ahead of the up some of the mechanical energy. This is most easily working piston by 90°. -
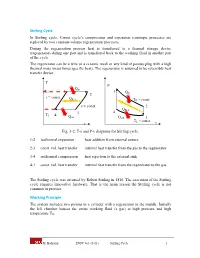
Stirling Cycle Working Principle
Stirling Cycle In Stirling cycle, Carnot cycle’s compression and expansion isentropic processes are replaced by two constant-volume regeneration processes. During the regeneration process heat is transferred to a thermal storage device (regenerator) during one part and is transferred back to the working fluid in another part of the cycle. The regenerator can be a wire or a ceramic mesh or any kind of porous plug with a high thermal mass (mass times specific heat). The regenerator is assumed to be reversible heat transfer device. T P Qin TH 1 1 Q 2 in v = const. Q Reg. TH = const. v = const. 2 QReg. 3 4 TL 4 Qout Qout 3 T = const. s L v Fig. 3-2: T-s and P-v diagrams for Stirling cycle. 1-2 isothermal expansion heat addition from external source 2-3 const. vol. heat transfer internal heat transfer from the gas to the regenerator 3-4 isothermal compression heat rejection to the external sink 4-1 const. vol. heat transfer internal heat transfer from the regenerator to the gas The Stirling cycle was invented by Robert Stirling in 1816. The execution of the Stirling cycle requires innovative hardware. That is the main reason the Stirling cycle is not common in practice. Working Principle The system includes two pistons in a cylinder with a regenerator in the middle. Initially the left chamber houses the entire working fluid (a gas) at high pressure and high temperature TH. M. Bahrami ENSC 461 (S 11) Stirling Cycle 1 TH TL QH State 1 Regenerator Fig 3-3a: 1-2, isothermal heat transfer to the gas at TH from external source. -

Design of a 2.5Kw Low Temperature Stirling Engine for Distributed Solar Thermal Generation
Design of a 2.5kW Low Temperature Stirling Engine for Distributed Solar Thermal Generation Mike He∗ and Seth Sandersy University of California - Berkeley, Berkeley, CA, 94720, USA This paper focuses on the design of a Stirling engine for distributed solar thermal ap- plications. In particular, we design for the low temperature differential that is attainable with distributed solar collectors and the low cost that is required to be competitive in this space. We will describe how these considerations drive the core design, the methodology for improving the design, and summarize progress made in fabrication of the engine for experimentation. Stirling engines can have broad significance and technological advantages for distributed renewable energy applications. A key advantage of a solar thermal system is that they can incorporate thermal energy storage in a cost-effective manner. In addition, Stirling engine systems are fuel-flexible with respect to the source of thermal energy and unprocessed waste heat can be harvested for CHP purposes as well. The ability to extract unconverted thermal energy for waste heat applications greatly improves the overall thermal efficiency of the system. I. Introduction enewable energy technology will need to address important challenges in order to be adopted at high Rpenetrations in a modern electric grid. These include achieving low enough cost to be economically attrac- tive and mitigating the variability inherent in renewable energy sources, a problem most directly addressed by energy storage. We propose a Stirling-engine-based solar thermal system for distributed generation of electricity as a renewable energy technology that addresses these challenges. The proposed system, as shown in Figure1, is comprised of a passive solar collector, a hot thermal storage subsystem, a Stirling engine for energy conversion, and a waste heat recovery system to implement combined heat and power.