Aerial Attack Strategies of Bat-Hunting Hawks, and the Dilution Effect of Swarming
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Birding Tour to Ghana Specializing on Upper Guinea Forest 12–26 January 2018
Birding Tour to Ghana Specializing on Upper Guinea Forest 12–26 January 2018 Chocolate-backed Kingfisher, Ankasa Resource Reserve (Dan Casey photo) Participants: Jim Brown (Missoula, MT) Dan Casey (Billings and Somers, MT) Steve Feiner (Portland, OR) Bob & Carolyn Jones (Billings, MT) Diane Kook (Bend, OR) Judy Meredith (Bend, OR) Leaders: Paul Mensah, Jackson Owusu, & Jeff Marks Prepared by Jeff Marks Executive Director, Montana Bird Advocacy Birding Ghana, Montana Bird Advocacy, January 2018, Page 1 Tour Summary Our trip spanned latitudes from about 5° to 9.5°N and longitudes from about 3°W to the prime meridian. Weather was characterized by high cloud cover and haze, in part from Harmattan winds that blow from the northeast and carry particulates from the Sahara Desert. Temperatures were relatively pleasant as a result, and precipitation was almost nonexistent. Everyone stayed healthy, the AC on the bus functioned perfectly, the tropical fruits (i.e., bananas, mangos, papayas, and pineapples) that Paul and Jackson obtained from roadside sellers were exquisite and perfectly ripe, the meals and lodgings were passable, and the jokes from Jeff tolerable, for the most part. We detected 380 species of birds, including some that were heard but not seen. We did especially well with kingfishers, bee-eaters, greenbuls, and sunbirds. We observed 28 species of diurnal raptors, which is not a large number for this part of the world, but everyone was happy with the wonderful looks we obtained of species such as African Harrier-Hawk, African Cuckoo-Hawk, Hooded Vulture, White-headed Vulture, Bat Hawk (pair at nest!), Long-tailed Hawk, Red-chested Goshawk, Grasshopper Buzzard, African Hobby, and Lanner Falcon. -

Progress in the Development of an Eurasian-African Bird Migration Atlas
CONVENTION ON UNEP/CMS/COP13/Inf.20 MIGRATORY 10 February 2020 SPECIES Original: English 13th MEETING OF THE CONFERENCE OF THE PARTIES Gandhinagar, India, 17 - 22 February 2020 Agenda Item 25 PROGRESS IN THE DEVELOPMENT OF AN EURASIAN-AFRICAN BIRD MIGRATION ATLAS (Submitted by the European Union of Bird Ringing (EURING) and the Institute of Avian Research) Summary: The African-Eurasian Bird Migration Atlas is being developed under the auspices of CMS in the framework of a Global Animal Migration Atlas, of which it constitutes a module. The African-Eurasian Bird Migration Atlas is being developed and compiled by the European Union of Bird Ringing (EURING) under a Project Cooperation Agreement (PCA) between the CMS Secretariat and the Institute of Avian Research, acting on behalf of EURING. The development of the African-Eurasian Bird Migration Atlas is funded with the contribution granted by the Government of Italy under the Migratory Species Champion Programme. This information document includes a progress report on the development of the various components of the project. The project is expected to be completed in 2021. UNEP/CMS/COP13/Inf.20 Eurasian-African Bird Migration Atlas progress report February 2020 Stephen Baillie1, Franz Bairlein2, Wolfgang Fiedler3, Fernando Spina4, Kasper Thorup5, Sam Franks1, Dorian Moss1, Justin Walker1, Daniel Higgins1, Roberto Ambrosini6, Niccolò Fattorini6, Juan Arizaga7, Maite Laso7, Frédéric Jiguet8, Boris Nikolov9, Henk van der Jeugd10, Andy Musgrove1, Mark Hammond1 and William Skellorn1. A report to the Convention on Migratory Species from the European Union for Bird Ringing (EURING) and the Institite of Avian Research, Wilhelmshaven, Germany 1. British Trust for Ornithology, Thetford, IP24 2PU, UK 2. -
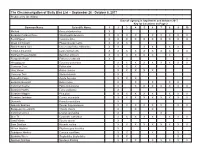
The Circumnavigation of Sicily Bird List -- September 26
The Circumnavigation of Sicily Bird List -- September 26 - October 8, 2017 Produced by Jim Wilson Date of sighting in September and October 2017 Key for Locations on Page 2 Common Name Scientific Name 1 2 3 4 5 6 7 8 9 10 Mallard Anas platyrhynchos X Eurasian Collared Dove Streptopelia decaocto X X X X X X X X X Feral Pigeon Columba livia X X X X X X X X X X Great Cormorant Phalacrocorax carbo X X X Black-headed Gull Chroicocephalus ridibundus X X X X X X Yellow-legged Gull Larus michahellis X X X X X X X X X X Northern House Martin Delichon urbicum X X X X X X X European Robin Erithacus rubecula X X Woodpigeon Columba palumbus X X X X X X X X X Common Coot Fulica atra X X X Grey Heron Ardea cinerea X X X X X Common Tern Sterna hirundo X Bonnelli's Eagle Aquila fasciata X X X Eurasian Buzzard Buteo buteo X X X X X Common Kestrel Falco tinnunculus X X X X X X X X Eurasian Hobby Falco subbuteo X Eurasian Magpie Pica pica X X X X X X X X Eurasian Jackdaw Corvus monedula X X X X X X X X X Dunnock Prunella modularis X Spanish Sparrow Passer hispaniolensis X X X X X X X X European Greenfinch Chloris chloris X X X Common Linnet Linaria cannabina X X Blue Tit Cyanistes caeruleus X X X X X X X X Sand Martin Riparia riparia X Barn Swallow Hirundo rustica X X X X X X X X X Willow Warbler Phylloscopus trochilus X Subalpine Warbler Curruca cantillans X X X X Eurasian Wren Troglodytes troglodytes X X X Spotless Starling Spotless Starling X X X X X X X X Common Name Scientific Name 1 2 3 4 5 6 7 8 9 10 Sardinian Warbler Curruca melanocephala X X X X -

HAVE the EGGS of the ORANGE&Hyphen;BREASTED
HAVE THE EGGS OF THE ORANGE-BREASTED FALCON (FALCO DEIROLEUCUS) BEEN DESCRIBED? by DouglasA. BoyceJr.' School of Natural Resources HumboldtState University Arcata, California 95521 and Lloyd F. Kiff WesternFoundation of VertebrateZoology 1100 Glendon Avenue LosAngeles, California 90024 The only publisheddescription of Orange-breastedFalcon (Falcodeiroleucus) eggs knownto us is that of Coltart (1952),who presenteddetails on two setsof eggssaid to be of thisspecies collected for G. D. Smookerin Trinidad.One of thesesets, a clutchof 3 takenon 28 March 1937in the Aripo Savannah,is now in the collectionof the West- ernFoundation of VertebrateZoology (WFVZ cat. no. 15,728);the other,a setof 2 col- lectedon 21 April 1930 in the Coroni Marshes,is in the collectionof the Zoological Museum,University of Helsinki,Finland (ZMUF cat. no. 15,721). The authenticityof theseeggs has been questioned by ffrench(1973) because of their smallsize compared to the bodysize of the species.The 3 eggsin the WFVZ collection measure40.6 x 34.7, 41.8 x 35.6, and 39.9 x 34.6 mm, and the 2 eggsin the ZMUF set measure 43.0 x 35.0 and 42.2 x 34.7 mm. All of these measurements fall within the rangegiven for eggsof the AplomadoFalcon (Falco femoralis) by Bent (1938) and Brownand Amadon(1968) and are only slightlylarger than the extrememeasurements knownfor eggsof the muchsmaller Bat Falcon(Falco rufigularis) (Brown and Amadon op cit., Kiff unpubl.data). Eggs of severalfalcon species, including one of Smooker's Trinidadeggs, are shownin Figurei to illustratetheir comparativesizes. Heinroth(1922) first demonstratedthe fundamentalrelationship between egg weight andbody weight in birds,and this was further refined by Huxley(1923-1924). -

Bird) Species List
Aves (Bird) Species List Higher Classification1 Kingdom: Animalia, Phyllum: Chordata, Class: Reptilia, Diapsida, Archosauria, Aves Order (O:) and Family (F:) English Name2 Scientific Name3 O: Tinamiformes (Tinamous) F: Tinamidae (Tinamous) Great Tinamou Tinamus major Highland Tinamou Nothocercus bonapartei O: Galliformes (Turkeys, Pheasants & Quail) F: Cracidae Black Guan Chamaepetes unicolor (Chachalacas, Guans & Curassows) Gray-headed Chachalaca Ortalis cinereiceps F: Odontophoridae (New World Quail) Black-breasted Wood-quail Odontophorus leucolaemus Buffy-crowned Wood-Partridge Dendrortyx leucophrys Marbled Wood-Quail Odontophorus gujanensis Spotted Wood-Quail Odontophorus guttatus O: Suliformes (Cormorants) F: Fregatidae (Frigatebirds) Magnificent Frigatebird Fregata magnificens O: Pelecaniformes (Pelicans, Tropicbirds & Allies) F: Ardeidae (Herons, Egrets & Bitterns) Cattle Egret Bubulcus ibis O: Charadriiformes (Sandpipers & Allies) F: Scolopacidae (Sandpipers) Spotted Sandpiper Actitis macularius O: Gruiformes (Cranes & Allies) F: Rallidae (Rails) Gray-Cowled Wood-Rail Aramides cajaneus O: Accipitriformes (Diurnal Birds of Prey) F: Cathartidae (Vultures & Condors) Black Vulture Coragyps atratus Turkey Vulture Cathartes aura F: Pandionidae (Osprey) Osprey Pandion haliaetus F: Accipitridae (Hawks, Eagles & Kites) Barred Hawk Morphnarchus princeps Broad-winged Hawk Buteo platypterus Double-toothed Kite Harpagus bidentatus Gray-headed Kite Leptodon cayanensis Northern Harrier Circus cyaneus Ornate Hawk-Eagle Spizaetus ornatus Red-tailed -

Syringeal Morphology and the Phylogeny of the Falconidae’
The Condor 96:127-140 Q The Cooper Ornithological Society 1994 SYRINGEAL MORPHOLOGY AND THE PHYLOGENY OF THE FALCONIDAE’ CAROLES.GRIFFITHS Departmentof Ornithology,American Museum of NaturalHistory and Departmentef Biology, City Collegeof City Universityof New York, Central Park West at 79th St., New York, NY 10024 Abstract. Variation in syringealmorphology was studied to resolve the relationshipsof representativesof all of the recognized genera of falcons, falconets, pygmy falcons, and caracarasin the family Falconidae. The phylogenyderived from thesedata establishesthree major cladeswithin the family: (1) the Polyborinae, containingDaptrius, Polyborus, Milvago and Phalcoboenus,the four genera of caracaras;(2) the Falconinae, consistingof the genus Falco, Polihierax (pygmy falcons),Spiziapteryx and Microhierax (falconets)and Herpetothe- res (Laughing Falcon); and (3) the genus Micrastur(forest falcons) comprising the third, basal clade. Two genera, Daptriusand Polihierax,are found to be polyphyletic. The phy- logeny inferred from these syringealdata do not support the current division of the family into two subfamilies. Key words: Falconidae;phylogeny; systematics; syrinx; falcons; caracaras. INTRODUCTION 1. The Polyborinae. This includes seven gen- Phylogenetic relationships form the basis for re- era: Daptrius, Milvago, Polyborus and Phalco- searchin comparative and evolutionary biology boenus(the caracaras),Micrastur (forest falcons), (Page1 and Harvey 1988, Gittleman and Luh Herpetotheres(Laughing Falcon) and Spiziapter- 1992). Patterns drawn from cladogramsprovide yx (Spot-winged Falconet). the blueprints for understanding biodiversity, 2. The Falconinae. This includes three genera: biogeography,behavior, and parasite-hostcospe- Falco, Polihierax (pygmy falcons) and Micro- ciation (Vane-Wright et al. 199 1, Mayden 1988, hierax (falconets). Page 1988, Coddington 1988) and are one of the Inclusion of the caracarasin the Polyborinae key ingredients for planning conservation strat- is not questioned (Sharpe 1874, Swann 1922, egies(Erwin 199 1, May 1990). -

Birds Versus Bats: Attack Strategies of Bat-Hunting Hawks, and the Dilution Effect of Swarming
Supplementary Information Accompanying: Birds versus bats: attack strategies of bat-hunting hawks, and the dilution effect of swarming Caroline H. Brighton1*, Lillias Zusi2, Kathryn McGowan2, Morgan Kinniry2, Laura N. Kloepper2*, Graham K. Taylor1 1Department of Zoology, University of Oxford, South Parks Road, Oxford, OX1 3PS, UK. 2Department of Biology, Saint Mary’s College, Notre Dame, IN 46556, USA. *Correspondence to: [email protected] This file contains: Figures S1-S2 Tables S1-S3 Supplementary References supporting Table S1 Legend for Data S1 and Code S1 Legend for Movie S1 Data S1 and Code S1 implementing the statistical analysis have been uploaded as Supporting Information. Movie S1 has been uploaded to figshare: https://doi.org/10.6084/m9.figshare.11823393 Figure S1. Video frames showing examples of attacks on lone bats and the column. (A,B) Attacks on the column of bats, defined as an attack on one or more bats within a cohesive group of individuals all flying in the same general direction. (C-E) Attacks on a lone bat (circled red), defined as an attack on an individual that appeared to be flying at least 1m from the edge of the column, and typically in a different direction to the swarm. (F) If an attack occurred in a volume containing many bats, but with no coherent flight direction, then this was also categorised as an attack on a lone bat, rather than as an attack on the swarm. Figure S2 Video frames used to estimate the proportion of bats meeting the criteria for classification as lone bats. -

Field Identification of Orange-Breasted and Bat Falcons
C O T IN G A 4 Orange-breasted and Bat falcons Field identification of Orange-breasted and Bat Falcons Steve N. G. Howell and Andrew Whittaker Introduction extent on chest of Bat Falcon); definite coarse The Bat Falcon Falco rufigularis is a wide buff barring on black vest (not fine white spread, small, generally quite common edging); and proportionately much stronger Neotropical falcon resident from Mexico to larger feet.” Other guides13,18 echo these fea South America, west of the Andes to Ecuador, tures, in particular the orange chest, buff east of the Andes to Brazil1. Over much of its barring on the narrower black chest band, and range the Bat Falcon is sympatric with the the proportionately larger feet of Orange larger and much rarer Orange-breasted Fal breasted. However, many Bat Falcons show con F. deiroleucus, which is resident from orangish to bright orange across the chest south-eastern Mexico to South America, east above the black vest (as cautioned by Ridgely of the Andes to Brazil1. These two species are & Gwynne16), the barring on the black vest can remarkably similar in plumage (Figure 1), but be whitish or buffy in both species, and judg the field identification of Orange-breasted, a ing the relative size of bill or feet on a lone near-threatened species6, is poorly treated in bird can be difficult. Becoming thoroughly many guides. Here we discuss characters that familiar with the structure and plumage vari can be used to help correctly identify Orange ation of the much commoner Bat Falcon is breasted Falcons. -

Migratory Birds of Ladakh a Brief Long Distance Continental Migration
WORLD'S MIGRATORY BIRDS DAY 08 MAY, 2021 B R O W N H E A D E D G U L L MIGRATORY BIRDS OF LADAKH A BRIEF LONG DISTANCE CONTINENTAL MIGRATION the Arctic Ocean and the Indian Ocean, and comprises several migration routes of waterbirds. It also touches “West Asian- East African Flyway”. Presence of number of high-altitude wetlands (>2500 m amsl altitude) with thin human population makes Ladakh a suitable habitat for migration and breeding of continental birds, including wetlands of very big size (e.g., Pangong Tso, Tso Moriri, Tso Kar, etc.). C O M M O N S A N D P I P E R Ladakh provides a vast habitat for the water birds through its complex Ladakh landscape has significance network of wetlands including two being located at the conjunction of most important wetlands (Tso Moriri, four zoogeographic zones of the world Tso Kar) which have been designated (Palearctic, Oriental, Sino-Japanese and as Ramsar sites. Sahara-Arabian). In India, Ladakh landscape falls in Trans-Himalayan Nearly 89 bird species (long distance biogeographic zone and two provinces migrants) either breed or roost in (Ladakh Mountains, 1A) and (Tibetan Ladakh, and most of them (59) are Plateau, 1B). “Summer Migrants”, those have their breeding grounds here. Trans-Himalayan Ladakh is an integral part of the "Central Asian Flyway" of migratory birds which a large part of the globe (Asia and Europe) between Ladakh also hosts 25 bird species, during their migration along the Central Asian Flyway, as “Passage Migrants” which roost in the region. -

Birding on the Roof of the World Promoting Low-Impact Nature Tourism for Conservation and Livelihoods in the Hindu Kush Karakoram Pamir Landscape
HKPL INITIATIVE Birding on the roof of the world Promoting low-impact nature tourism for conservation and livelihoods in the Hindu Kush Karakoram Pamir Landscape Photo: Imran Shah Landscape ecology and biodiversity The region’s forests, wetlands, The transboundary Hindu Kush Karakoram Pamir rangelands, and peatlands serve as Landscape (HKPL) – a biodiversity-rich location spanning natural habitat for a wide variety of parts of Afghanistan, China, Pakistan, and Tajikistan – is home to six protected areas: the Wakhan National Park resident and migratory birds. of Afghanistan; the Taxkorgan Nature Reserve of China; the Broghil, Qurumber, and Khunjerab national parks of Pakistan; and the Zorkul Nature Reserve of Tajikistan. birds. The landscape is an important migratory corridor, Together, these physically connected protected areas cover hosting staging and breeding grounds for several species 33,000 square kilometres, just under half of the total area of including the Critically Endangered sociable lapwing and the the landscape. Endangered steppe eagle, saker falcon, and Pallas’s fish eagle. As part of the western Hindu Kush Himalaya, the HKPL lies As ecological indicators of habitat quality, birds provide at a junction of several important biogeographical regions. important information about ecosystem health for A unique diversity of flora and fauna, including 306 bird conservation planning, setting management priorities, and species, flourish in its cold desert ecosystem. measuring the success of restoration efforts. In the HKPL, Birds are an integral part of the HKPL’s biodiversity. The the six contiguous protected areas present opportunities for region’s wetlands, rangelands, and peatlands are the transboundary cooperation for biodiversity conservation and natural habitat of a wide variety of resident and migratory sustainable development. -

Project Name
SYRAH RESOURCES GRAPHITE PROJECT, CABO DELGADO, MOZAMBIQUE TERRESTRIAL FAUNAL IMPACT ASSESSMENT Prepared by: Prepared for: Syrah Resources Limited Coastal and Environmental Services Mozambique, Limitada 356 Collins Street Rua da Frente de Libertação de Melbourne Moçambique, Nº 324 3000 Maputo- Moçambique Australia Tel: (+258) 21 243500 • Fax: (+258) 21 243550 Website: www.cesnet.co.za December 2013 Syrah Final Faunal Impact Assessment – December 2013 AUTHOR Bill Branch, Terrestrial Vertebrate Faunal Consultant Bill Branch obtained B.Sc. and Ph.D. degrees at Southampton University, UK. He was employed for 31 years as the herpetologist at the Port Elizabeth Museum, and now retired holds the honorary post of Curator Emeritus. He has published over 260 scientific articles, as well as numerous popular articles and books. The latter include the Red Data Book for endangered South African reptiles and amphibians (1988), and co-editing its most recent upgrade – the Atlas and Red Data Book of the Reptiles of South Africa, Lesotho and Swaziland (2013). He has also published guides to the reptiles of both Southern and Eastern Africa. He has chaired the IUCN SSC African Reptile Group. He has served as an Honorary Research Professor at the University of Witwatersrand (Johannesburg), and has recently been appointed as a Research Associate at the Nelson Mandela Metropolitan University, Port Elizabeth. His research concentrates on the taxonomy, biogeography and conservation of African reptiles, and he has described over 30 new species and many other higher taxa. He has extensive field work experience, having worked in over 16 African countries, including Gabon, Ivory Coast, DRC, Zambia, Mozambique, Malawi, Madagascar, Namibia, Angola and Tanzania. -

Red Necked Falcon
Ca Identifi cation Habit: The Red-necked Falcon is an arboreal and Features: Cultural Aspects: aerial crepuscular bird. Lives and hunts in pairs. In ancient India this falcon was Flight is fast and straight. It is capable of hovering. esteemed by falconers as it hunts in 1st and 4th primary pairs, is easily trained and is obedient. Distributation: India upto Himalayan foothills and subequal. 2nd and 3rd It took birds as large as partridges. terrai; Nepal, Pakistan and BanglaDesh. South of primary subequal. In ancient Egypt, Horus, was the Sahara in Africa. Crown and cheek stripe falcon-headed god of sun, war and chestnut. protection and was associated with Habitat: Keeps to plain country with deciduous the Pharoahs. vegetation, hilly terrain, agricultural cropland with Bill plumbeous, dark groves, semiarid open scrub country and villages. tipped. Avoids forests. Iris brown. Related Falcons: Behaviour: Resident falcon with seasonal Cere, orbital skin, legs and Common Kestrel, Shaheen and Laggar Falcon are residents . The movements that are not studied. Swiftly chases feet yellow. Peregrine, Eurasian Hobby and Merlin are migrants. Red-legged crows, kites and other raptors that venture near its Falcon is extra-limital and is not recorded from India. nest. Shrill call is uttered during such frantic chase. Utters shrill and piercing screams ki ki ki ki, with diff erent calls, grates and trills for other occasions. Female feeds the male during the breeding season. A pair at sunrise roosting on the topmost perches of a tall tree Claws black. Tail broad with black sub-terminal band. Thinly barred abdomen MerlinM Common Kestrel Laggar Falcon Amur Falcon and fl anks.