VISIPAQUE EIRE Abbreviated Prescribing Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Original Article the Imaging Characteristics of Magnetic Resonance Hysterosalpingography in Infertile Women
Int J Clin Exp Med 2020;13(6):3955-3962 www.ijcem.com /ISSN:1940-5901/IJCEM0107779 Original Article The imaging characteristics of magnetic resonance hysterosalpingography in infertile women Jiugen Ruan1, Chunyan Wu2, Changhua Zhu1, Yao Ding1, Qiuping Tan1, Tao Meng3 Departments of 1Radiology, 2Gynaecology, The People’s Hospital of Xinyu City, Xinyu, Jiangxi, China; 3RIMAG Medical Imaging Corporation, Shanghai, China Received January 13, 2020; Accepted April 1, 2020; Epub June 15, 2020; Published June 30, 2020 Abstract: Objective: We aimed to analyze the imaging characteristics of magnetic resonance hysterosalpingography (MR-HSG) in infertile women. Methods: A total of 20 infertile women admitted to our hospital from October 2018 to December 2019 were selected as the subjects of study for retrospective analysis. The MR-HSG examination was performed in all patients to analyze the examination results and imaging characteristics. Results: (1) The comple- tion rate of MR-HSG examination was 100.00% in the 20 patients, of which those with primary infertility accounted for 65.00% and those with secondary infertility accounted for 35.00%. (2) Among the 20 infertile patients, 30.00% had unobstructed fallopian tubes, 40.00% had partial fallopian tube obstruction, 20.00% had full fallopian tube obstruction and 10.00% had hydrosalpinx. (3) Among the 14 patients with abnormal fallopian tubes, 14.29% had bilateral fallopian tube obstruction, 35.71% had partial fallopian tube obstruction on both sides, 7.14% had fallo- pian tubes that were unobstructed on one side and obstructed on the other side, 21.43% had fallopian tubes that were unobstructed on one side and partially obstructed on the other side, 7.14% had fallopian tubes partially ob- structed on one side and obstructed on the other side, and 14.29% had hydrosalpinx. -

Hysterosalpingography
H y s t e r o s alp i n g o g r aph y A b o u t P r e p a r a t i o n s Hysterosalpingography is an x-ray examination of the The hysterosalpingography procedure is best uterus and fallopian tubes that uses a special form of x- performed one week after menstruation but before ray called fluoroscopy and a contrast material. During a ovulation to make certain that you are not pregnant hysterosalpingogram, the uterus and fallopian tubes are during the exam. This procedure should not be filled with a contrast material and the radiologist is able to performed if you have an active inflammatory condition. use fluoroscopy to view and assess their anatomy and You should notify your radiologist if you have a chronic function. pelvic infection or an untreated sexually transmitted disease at the time of the procedure. Hysterosalpingography is primarily used to examine women who have difficulty becoming pregnant by On the night before the procedure, you may be asked allowing the radiologist to evaluate the shape and to take a laxative or an enema to empty your bowels, so structure of the uterus, the openness of the fallopian the uterus and surrounding structures can be seen tubes, and any scarring within the uterine or abdominal clearly. Prior to the procedure, you may be given a mild cavity. sedative or over-the-counter medication to minimize any potential discomfort. The exam is used to investigate repeated miscarriages that result from abnormalities in the uterus and to You should inform your radiologist of any medications determine the presence and severity of the following you are taking and if you have any allergies, especially abnormalities: to barium or iodinated contrast materials. -

Procedure Codes for Physician: Radiology
NEW YORK STATE MEDICAID PROGRAM PHYSICIAN - PROCEDURE CODES SECTION 4 - RADIOLOGY Physician – Procedure Codes, Section 4 - Radiology Table of Contents GENERAL INSTRUCTIONS ............................................................................................................ 4 GENERAL RULES AND INFORMATION ......................................................................................... 6 MMIS RADIOLOGY MODIFIERS .................................................................................................... 8 DIAGNOSTIC RADIOLOGY (DIAGNOSTIC IMAGING)................................................................. 9 HEAD AND NECK.................................................................................................................... 9 CHEST .................................................................................................................................. 10 SPINE AND PELVIS .............................................................................................................. 11 UPPER EXTREMITIES .......................................................................................................... 12 LOWER EXTREMITIES ......................................................................................................... 13 ABDOMEN ............................................................................................................................ 14 GASTROINTESTINAL TRACT ............................................................................................... 15 URINARY -

Zwanger-Pesiri Radiology Provides the Latest Ability to Detect, Characterize, Stage Ultrasound (Sonography) Equipment at Each of and Treat Disease Our Offices
ESIRI P At the Forefront of Forefront the At WANGER- Z Technology, Diagnosis & Care Technology, 521 Route 111 Stony Brook Medical Park Hauppauge 11788 2500 Nesconset Hwy, Bldg 15 Stony Brook 11790 80 Maple Avenue 25A Smithtown 11787 220 Belle Mead Rd 763 Larkfield Road R Commack 11725 347 East Setauket 11733 - E 25A W - 680 Old Country Rd i 25A l l i 2087 Deer Park Ave a Plainview 11803 25 m Deer Park 11729 N G i F c 112 l 1500 William Floyd Pkwy - 111 o o y l l d s Shirley 11967 272 North Broadway kwy P P 25 R 495 k N Hicksville 11801 the t e d w r rn - No Sta 231 y S 495 a g 111 454 27 A t i 495 k o 1390 Hempstead Tpke Suffolk Ave - s Brookhaven Prof. Park 11003 Wantagh Pkwy P Elmont y M k w 135 106 w k ead North Ocean Plaza 285 Sills Road, Bldg 15 o kwy y P Hempstead Tpke P W w 107 te ta b S 1729 N. Ocean Ave Patchogue 11772 d n - r r n e o a South 443 Sunrise Hwy l 24 o 27 Medford 11763 s k I Z zprad.com Lynbrook 11563 s P s k 27A o r w lt Pkwy C y Be S nrise u Hwy 759 Montauk Hwy 160 Brentwood Rd 2012 Sunrise Hwy West Islip 11795 Bay Shore 11706 516 631 Merrick 11566 126 Hicksville Rd Massapequa 11758 150 E. Sunrise Hwy Lindenhurst 11757 CVS Plaza 355 Broadway Amityville 11701 C ontinuum of C are Echocardiography Two-dimensional imaging of the cardiovascular system Confidence Accurate assessment of the velocity Unsurpassed diagnostic technology of blood, cardiac tissue and cardiac valves The most experience with 3.0 Tesla MRI and 3D Mammography on Long Island Visualizes leaking of blood through the valves (valvular regurgitation) -

ACR–ACOG–AIUM–SRU PRACTICE PARAMETER for the PERFORMANCE of SONOHYSTEROGRAPHY and HYSTEROSALPINGO- CONTRAST-SONOGRAPHY (Hycosy)
The American College of Radiology, with more than 30,000 members, is the principal organization of radiologists, radiation oncologists, and clinical medical physicists in the United States. The College is a nonprofit professional society whose primary purposes are to advance the science of radiology, improve radiologic services to the patient, study the socioeconomic aspects of the practice of radiology, and encourage continuing education for radiologists, radiation oncologists, medical physicists, and persons practicing in allied professional fields. The American College of Radiology will periodically define new practice parameters and technical standards for radiologic practice to help advance the science of radiology and to improve the quality of service to patients throughout the United States. Existing practice parameters and technical standards will be reviewed for revision or renewal, as appropriate, on their fifth anniversary or sooner, if indicated. Each practice parameter and technical standard, representing a policy statement by the College, has undergone a thorough consensus process in which it has been subjected to extensive review and approval. The practice parameters and technical standards recognize that the safe and effective use of diagnostic and therapeutic radiology requires specific training, skills, and techniques, as described in each document. Reproduction or modification of the published practice parameter and technical standard by those entities not providing these services is not authorized. Revised 2020 (Resolution 4) * ACR–ACOG–AIUM–SRU PRACTICE PARAMETER FOR THE PERFORMANCE OF SONOHYSTEROGRAPHY AND HYSTEROSALPINGO- CONTRAST-SONOGRAPHY (HyCoSy) PREAMBLE This document is an educational tool designed to assist practitioners in providing appropriate radiologic care for patients. Practice Parameters and Technical Standards are not inflexible rules or requirements of practice and are not intended, nor should they be used, to establish a legal standard of care1. -

Kidney Ureter Bladder Diagnostic Technique
KUB Kidney Ureter Bladder Diagnostic Technique . Plain KUB . Intravenous urography : IVP . Voiding cystourethrography : VCUG . Hysterosalpingography . Ultrasonography Plain KUB . The projection includes the entire urinary system . From the superior aspects of the kidneys . To the pubic symphysis . Not include the diaphragm Indication Plain films are widely used in the management of stone diseases What are you looking for? . Calcifications . Abnormal soft tissue . Air within urinary tract . Bony abnormalities Transverse Process Urinary Tract Stone . Most common cause of acute ureteral obstruction . Clinical presentation : 1. Flank pain 2. Intermittent hematuria Clinical Perspective Acute flank pain, with dramatic relief upon passage of the stone 1. UPJ stone : flank pain 2. Proximal ureteric stone : flank pain radiating to the genitals 3. UVJ stone : voiding urgency and suprapubic discomfort, and they cause pain that radiates into the groin and genitals Hematuria Radiologic Evaluation of Hematuria . The AUA guidelines recommended upper tract imaging for low- and high-risk patients with microscopic hematuria . Gross hematuria -> much higher risk of malignancy than microscopic disease Hematuria : Cause . Calculi . Infection . Cancer . Obstruction . Anticoagulation . Artifactual cause: menstrual blood, food such as beets, berries, rhubarb Radiologic Work-up of Hematuria . No universal agreement about the optimal imaging work-up of hematuria . Traditionally : IVP was the standard . Recently : . Multidetector CT scans have become routine . MRI can be used to detect urinary tract abnormalities, but has limited use because of its expense and the lack of data supporting its use ACR Appropriateness Criteria Scale for Hematuria ACR Appropriateness Criteria Scale for Hematuria ACR Appropriateness Criteria Scale for Hematuria Urinary tract stone . Increasing overall prevalence in the last decade . -

Magnetic Resonance Hysterosalpingography in Diagnostic Work-Up of Female Infertility – Comparison with Conventional Hysterosalpingography: a Randomised Study
European Radiology (2019) 29:501–508 https://doi.org/10.1007/s00330-018-5572-2 UROGENITAL Magnetic resonance hysterosalpingography in diagnostic work-up of female infertility – comparison with conventional hysterosalpingography: a randomised study Manuelle Volondat1 & Eric Fontas2 & Jerome Delotte3 & Imene Fatfouta3 & Patrick Chevallier1 & Madleen Chassang1 Received: 17 January 2018 /Revised: 17 May 2018 /Accepted: 28 May 2018 /Published online: 4 July 2018 # European Society of Radiology 2018 Abstract Objective To compare diagnostic accuracy of MR-hysterosalpingography (MR-HSG) and conventional hysterosalpingography (X-HSG) in the evaluation of female infertility. Methods Forty women received prospectively both X-HSG, the gold standard technique, and MR-HSG on the same day but the order in which they were conducted was randomised. A 1.5 Tesla MRI was performed with classical sequences for pelvic analysis and an additional 3D T1-weighted sequence with intra-uterine injection of gadolinium. Two radiologists independently interpreted X-HSG and MR-HSG according to randomisation, blinded to the other results. They both then performed a second interpretation of MR-HSG blinded to the first reading with a minimum time delay of 1 week. Diagnostic performance of MR-HSG for analysis of tubal and intracavity abnormalities was evaluated by calculating sensitivity (Se), specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV). Results Twenty-six patients were included. Diagnostic performance of MR-HSG was: Se: 91.7% (95% CI 61.5–99.8); Sp: 92.9% (95% CI 66.1–99.8) ; PPV: 91.7% (95% CI 61.5–99.8); NPV: 92.9% (95% CI 66.1–99.8). -

Hysterosal Pi Ngography: Current Applications Eng C W, Tang P H, Ong C L
Pictorial Essay Singapore Med 12007, 48 (4) : 368 CME Article Hysterosal pi ngography: current applications Eng C W, Tang P H, Ong C L ABSTRACT With the recent advances in reproductive medicine, hysterosalpingography has become a relatively quick and non- invasive examination to evaluate fallopian tubes and uterine cavity. It remains the best modality to image fallopian tubes. Congenital uterine malformations, technical artefacts and pathological findings are depicted. Pathological findings that can be detected on hysterosalpingography include salpingitis isthmica nodosa, tubal blockage, Fig. I Normal hysterosalpingogram shows a smooth triangular outline of the uterine cavity and free spillage of contrast from peritubal adhesion, submucosal leiomyoma, both fallopian tubes. endometrial polyp, endometrial carcinoma, synechiae and adenomyosis. Keywords: fallopian tube, hysterosalpingo- a smooth triangular uterine outline with opacification graphy, reproductive medicine, uterus of both fallopian tubes and free spillage of contrast Singapore Med J 2007; 48(4):368-374 into the peritoneum (Fig. 1). Occasionally, in difficult cases, the Leech -Wilkinson or other uterine catheters INTRODUCTION may be used. With the recent advances in reproductive medicine, hysterosalpingography has become a relative quick TECHNICAL ARTIFACTS and non-invasive examination to evaluate the fallopian Air bubbles tubes and uterine cavity. It remains the best modality to Air bubbles are often inadvertently introduced into image the fallopian tubes. Radiation risks from a typical the uterine cavity during hysterosalpingography. hysterosalpingography are also low.'" In addition, there When multiple air bubbles are present, they can be is also a controversial point that the procedure may be easily recognised. However, a single air bubble can be therapeutic.'2'3' The purpose of this pictorial essay is to mistaken for other uterine pathologies, such as a polyp illustrate the spectrum of technical artefacts, congenital or a submucosal fibroid. -
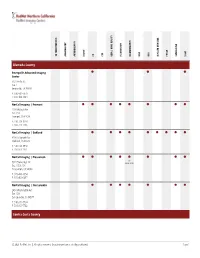
Service Grid
ANGIOGRAPHY CT DEXA | BONE DENSITY FLUOROSCOPY MRA PET/CT X-RAY 3D TOMOSYNTHESIS ARTHROGRAPHY BIOPSY CTA MAMMOGRAPHY MRI NUCLEAR MEDICINE ULTRASOUND Alameda County ⬤ ⬤ ⬤ Emeryville Advanced Imaging Center 6121 Hollis St. Ste. 1 Emeryville, CA 94608 P: 510-601-7979 F: 510-420-3484 NorCal Imaging | Fremont ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ 2201 Walnut Ave. Ste. 150 Fremont, CA 94538 P: 510-713-1234 F: 510-713-1236 NorCal Imaging | Oakland ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ 3200 Telegraph Ave. Oakland, CA 94609 P: 510-663-1950 F: 510-663-1951 NorCal Imaging | Pleasanton ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ 3D 5924 Stoneridge Dr. Mammography Ste. 105 & 106 Pleasanton, CA 94588 P: 925-463-0554 F: 925-463-0497 NorCal Imaging | San Leandro ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ 2450 Washington Ave. Ste. 120 San Leandro, CA 94577 P: 510-351-7734 F: 510-351-7742 Contra Costa County © 2021 RadNet, Inc. | All rights reserved. Unauthorized use is strictly prohibited. Page 1 ANGIOGRAPHY CT DEXA | BONE DENSITY FLUOROSCOPY MRA PET/CT X-RAY 3D TOMOSYNTHESIS ARTHROGRAPHY BIOPSY CTA MAMMOGRAPHY MRI NUCLEAR MEDICINE ULTRASOUND NorCal Imaging | Concord ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ 2300 Clayton Rd. Ste. 160 Concord, CA 94520 P: 925-825-7777 F: 925-288-8719 NorCal Imaging | Walnut Creek ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ 114 La Casa Via Ste. 100 & 200 Walnut Creek, CA 94598 P: 925-937-6100 F: 925-938-9940 Sacramento County ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ Diagnostic Radiological Imaging Elk Grove Digital Mammography 7911 Laguna Blvd. Elk Grove, CA 95758 P: 916-585-8990 F: 916-478-3710 ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ ⬤ Diagnostic Radiological Imaging Sacramento Digital Mammography 79 Scripps Dr. Ste. 100 Sacramento, CA 95825 P: 916-921-1300 F: 916-921-1090 San Francisco County ⬤ ⬤ ⬤ ⬤ RadNet Medical Imaging | San Francisco 3440 California St. San Francisco, CA 94118 P: 415-922-6767 F: 415-563-0468 San Joaquin County © 2021 RadNet, Inc. -

Urografin Data Sheet
DATA SHEET UROGRAFIN® (Sodium amidotrizoate/ Amidotrizoate meglumine) 1. NAME OF THE MEDICINE Sodium amidotrizoate / Amidotrizoate meglumine 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Urografin 30% contains 40 mg/mL sodium amidotrizoate and 260 mg/mL amidotrizoate meglumine in aqueous solution. Urografin 76% contains 100 mg/mL sodium amidotrizoate and 660 mg/mL amidotrizoate meglumine in aqueous solution. For the full list of excipients, see Section 6.1 List of excipients. 3. PHARMACEUTICAL FORM Solution for injection or infusion. 4. CLINICAL PARTICULARS 4.1 THERAPEUTIC INDICATIONS Intravenous and retrograde urography. Also for all angiographic examinations as well as for amniography, arthrography, intraoperative cholangiography, endoscopic retrograde cholangiopancreatography (ERCP), sialography, fistulography, hysterosalpingography, splenoportography, vesiculography and others. Urografin is not to be used for myelography, ventriculography or cisternography, since it is likely to provoke neurotoxic symptoms in these examinations. 4.2 DOSE AND METHOD OF ADMINISTRATION General Information Dietary suggestions In the case of abdominal angiography and urography, the diagnostic yield is increased if the bowels are emptied of faecal matter and gas. On the two days prior to the examination, patients should therefore avoid flatulent food, in particular peas, beans, lentils, salads, fruit, dark and fresh bread and all kinds of uncooked vegetables. On the day before the examination, patients should refrain from eating after 6 pm. Moreover, it can be appropriate to administer a laxative in the evening. In babies and young children, however, prolonged fasting and the administration of a laxative before the examination are contraindicated. Hydration Adequate hydration must be assured before and after contrast medium administration. This applies especially to patients with multiple myeloma, diabetes mellitus with nephropathy, polyuria, oliguria, hyperuricaemia, as well as to newborns, infants, small children and elderly patients. -

Original Article the Water-Soluble Iodinated Contrast Medium Used For
Int J Clin Exp Med 2016;9(10):20008-20013 www.ijcem.com /ISSN:1940-5901/IJCEM0023636 Original Article The water-soluble iodinated contrast medium used for hysterosalpingography might increase the probability of development of subclinical thyroid diseases Umit Gorkem1, Ferit Kerim Kucukler2, Ramazan Kocabas3, Hasan Ali Inal4, Nafiye Yilmaz5, Ayla Sargin Oruc5, Tayfun Gungor1 1Hitit University Faculty of Medicine, Obstetrics and Gynecology, Corum, Turkey; 2Hitit University Faculty of Medi- cine, Endocrinology, Corum, Turkey; 3Hitit University Faculty of Medicine, Biochemistry, Corum, Turkey; 4Konya Edu- cation and Research Hospital, Obstetrics and Gynecology, Konya, Turkey; 5Zekai Tahir Burak Maternal Education and Research Hospital, Obstetrics and Gynecology, Ankara, Turkey Received January 9, 2016; Accepted May 18, 2016; Epub October 15, 2016; Published October 30, 2016 Abstract: Objective: Sudden exposure to high iodide levels may cause thyroid dysfunction. Although the iodinated contrast medium used for computed tomography and coronary angiography is known to alter thyroid function, the water-soluble iodinated contrast medium (ICM) used for hysterosalpingography (HSG) has not been investigated comprehensively. In the present study, we aimed to investigate the effect of the water-soluble iodinated contrast medium used for HSG on thyroid functional tests in euthyroid infertile women. Materials and methods: A total of 87 euthyroid infertile women with normal thyroid stimulating hormone (TSH), triiodothyronin (fT3) and free thyroxine (fT4) levels were included in the study. Serum TSH, fT4, and fT3 levels were measured before the study and one week, one month, and three months after HSG. Patients underwent a thyroid ultrasonography to reveal the occur- rence of any change. Results: Mean TSH levels did not change significantly until the 3rd month (P=0.03). -

Diagnostic Imaging and Cataloguing of Female Genital Malformations
Insights Imaging (2016) 7:713–726 DOI 10.1007/s13244-016-0515-4 Diagnostic imaging and cataloguing of female genital malformations Pedro Acién1,2,3,4 & Maribel Acién1,2,3 Received: 3 May 2016 /Revised: 13 July 2016 /Accepted: 20 July 2016 /Published online: 9 August 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract malformative combinations, all of them complex To help physicians and radiologists in the diagnosis of female malformations. Diagnostic imaging for all these anomalies is genito-urinary malformations, especially of complex cases, essential. The best imaging tools and when to evaluate for the embryology of the female genital tract, the basis for other anomalies are also analysed in this review. Müllerian development anomalies, the current classifications for such anomalies and the comparison for inclusion and Teaching points cataloguing of female genital malformations are briefly • The appropriate cataloguing of female genital reviewed. The use of the embryological system to catalogue malformations is controversial. female genito-urinary malformations may ultimately be more • An embryological classification system suggests the best useful in correlations with clinical presentations and in help- diagnosis and appropriate management. ing with the appropriate diagnosis and treatment. Diagnostic • The anomalies most frequently diagnosed incorrectly are the imaging of the different genito-urinary anomalies are exposed, distal mesonephric anomalies (DMAs). placing particular emphasis on the anomalies within group II • DMAs are associated with unilateral renal agenesis or renal of the embryological and clinical classification (distal meso- dysplasia with ectopic ureter. nephric anomalies), all of them associated with unilateral renal • We analyse other complex malformations.