What Is New on Ovarian Carcinoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ovarian Cancer and Cervical Cancer
What Every Woman Should Know About Gynecologic Cancer R. Kevin Reynolds, MD The George W. Morley Professor & Chief, Division of Gyn Oncology University of Michigan Ann Arbor, MI What is gynecologic cancer? Cancer is a disease where cells grow and spread without control. Gynecologic cancers begin in the female reproductive organs. The most common gynecologic cancers are endometrial cancer, ovarian cancer and cervical cancer. Less common gynecologic cancers involve vulva, Fallopian tube, uterine wall (sarcoma), vagina, and placenta (pregnancy tissue: molar pregnancy). Ovary Uterus Endometrium Cervix Vagina Vulva What causes endometrial cancer? Endometrial cancer is the most common gynecologic cancer: one out of every 40 women will develop endometrial cancer. It is caused by too much estrogen, a hormone normally present in women. The most common cause of the excess estrogen is being overweight: fat cells actually produce estrogen. Another cause of excess estrogen is medication such as tamoxifen (often prescribed for breast cancer treatment) or some forms of prescribed estrogen hormone therapy (unopposed estrogen). How is endometrial cancer detected? Almost all endometrial cancer is detected when a woman notices vaginal bleeding after her menopause or irregular bleeding before her menopause. If bleeding occurs, a woman should contact her doctor so that appropriate testing can be performed. This usually includes an endometrial biopsy, a brief, slightly crampy test, performed in the office. Fortunately, most endometrial cancers are detected before spread to other parts of the body occurs Is endometrial cancer treatable? Yes! Most women with endometrial cancer will undergo surgery including hysterectomy (removal of the uterus) in addition to removal of ovaries and lymph nodes. -

About Ovarian Cancer Overview and Types
cancer.org | 1.800.227.2345 About Ovarian Cancer Overview and Types If you have been diagnosed with ovarian cancer or are worried about it, you likely have a lot of questions. Learning some basics is a good place to start. ● What Is Ovarian Cancer? Research and Statistics See the latest estimates for new cases of ovarian cancer and deaths in the US and what research is currently being done. ● Key Statistics for Ovarian Cancer ● What's New in Ovarian Cancer Research? What Is Ovarian Cancer? Cancer starts when cells in the body begin to grow out of control. Cells in nearly any part of the body can become cancer and can spread. To learn more about how cancers start and spread, see What Is Cancer?1 Ovarian cancers were previously believed to begin only in the ovaries, but recent evidence suggests that many ovarian cancers may actually start in the cells in the far (distal) end of the fallopian tubes. 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 What are the ovaries? Ovaries are reproductive glands found only in females (women). The ovaries produce eggs (ova) for reproduction. The eggs travel from the ovaries through the fallopian tubes into the uterus where the fertilized egg settles in and develops into a fetus. The ovaries are also the main source of the female hormones estrogen and progesterone. One ovary is on each side of the uterus. The ovaries are mainly made up of 3 kinds of cells. Each type of cell can develop into a different type of tumor: ● Epithelial tumors start from the cells that cover the outer surface of the ovary. -

Pure Choriocarcinoma of the Ovary: a Case Report
Case Report J Gynecol Oncol Vol. 22, No. 2:135-139 pISSN 2005-0380 DOI:10.3802/jgo.2011.22.2.135 eISSN 2005-0399 Pure choriocarcinoma of the ovary: a case report Lin Lv1, Kaixuan Yang2, Hai Wu1, Jiangyan Lou1, Zhilan Peng1 Departments of 1Obstetrics and Gynecology and 2Pathology, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China Pure ovarian choriocarcinomas are extremely rare and aggressive tumors which are gestational or nongestational in origin. Due to the rarity of the tumor, there is a lack of information on the clinicopathologic features, diagnosis, and treatment. We report a case of a pure ovarian choriocarcinoma, likely of nongestational origin, treated by cytoreductive surgery in combination with postoperative chemotherapy. The patient was free of disease after a 12month followup. Keywords: Choriocarcinoma, Nongestational, Ovary INTRODUCTION CASE REPORT Pure ovarian choriocarcinomas are extremely rare malignan A 48yearold woman was admitted to our department cies which are of gestational or nongestational in origin. with a 6month history of irregular vaginal bleeding and a The gestational type may arise from an ectopic ovarian pre 1month history of a palpable abdominal mass. She had a gnancy or present as a metastasis from a uterine or tubal nor mal vaginal delivery at 26 years of age and had no recent choriocarcinoma, while the nongestational type is a rare history of normal pregnancies, molar gestations, or abortions. germ cell tumor with trophoblastic differentiation. The esti The physical examination revealed abdominal tenderness and mated incidence of gestational ovarian choriocarcinomas a fixed mass arising from the pelvis to 3 cm below the um is 1 in 369 million pregnancies [1]. -

Photodynamic Therapy for Gynecological Diseases and Breast Cancer
CancCancerer Biol Med 2012;2012 /9: Vol. 9-17 9 /doi: No. 10.3969/j.issn.2095-3941.2012.1 01.002 9 Review Photodynamic Therapy for Gynecological Diseases and Breast Cancer Natashis Shishkova, Olga Kuznetsova, Temirbolat Berezov Department of Biochemistry, School of Medicine, People’s Friendship University of Russia, Moscow 117198, Russia ABSTRACT Photodynamic therapy (PDT) is a minimally invasive and promising new method in cancer treatment. Cytotoxic reactive oxygen species (ROS) are generated by the tissue-localized non-toxic sensitizer upon illumination and in the presence of oxygen. Thus, selective destruction of a targeted tumor may be achieved. Compared with traditional cancer treatment, PDI has advantages including higher selectivity and lower rate of toxicity. The high degree of selectivity of the proposed method was applied to cancer diagnosis using fluorescence. This article reviews previous studies done on PDT treatment and photodetection of cervical intraepithelial neoplasia, vulvar intraepithelial neoplasia, ovarian and breast cancer, and PDT application in treating non-cancer lesions. The article also highlights the clinical responses to PDT, and discusses the possibility of enhancing treatment efficacy by combination with immunotherapy and targeted therapy. KEY WORDS: photodynamic therapy, photosensitizers, cervical/vulvar intraepithelial neoplasia, ovarian neoplasms, breast neoplasms Introduction frequently used drug in PDT is 5-aminolaevulinic acid (ALA). However, 5-ALA is not a photosensitizer, but a precursor of Photodynamic therapy (PDT) is a mode of therapy used in the endogenous photosensitizer protoporphyrin IX, which is cancer treatment where drug activity is locally controlled by a member of the heme synthesis pathway that occurs in the light (Figure 1). -

ASCO Answers: Ovarian, Fallopian Tube, and Peritoneal Cancer
Ovarian, Fallopian Tube & Peritoneal Cancer What are ovarian, fallopian tube, and peritoneal cancers? The term “ovarian cancer” is often used to describe cancers that begin in the cells in the ovary, fallopian tube, and peritoneum. These types of cancer begin when healthy cells in these areas change and grow out of control, forming a mass called a tumor. Research suggests that high-grade serous cancer, which includes most ovarian cancer, usually starts in the fallopian tubes. Some peritoneal cancers also may begin in the fallopian tube. What are the functions of the ovaries, fallopian tubes, and peritoneum? The ovaries and fallopian tubes are part of a woman’s reproductive system. Typically, every woman has 2 ovaries, which contain eggs and are the primary source of estrogen and progesterone. These hormones play a role in breast growth, body shape, body hair, the menstrual cycle, and pregnancy. ONCOLOGY. CLINICAL AMERICAN SOCIETY OF 2004 © LLC. EXPLANATIONS, MORREALE/VISUAL ROBERT BY ILLUSTRATION There are 2 fallopian tubes, which are small ducts that link the ovaries to the uterus. During a woman’s monthly ovulation, an egg is usually released from 1 ovary and travels through the fallopian tube to the uterus. The peritoneum is a tissue that lines the abdomen and most of the organs in the abdomen. What do stage and grade mean? Staging is a way of describing a cancer’s location, if or where it has spread, and whether it is affecting other parts of the body. There are 4 stages for ovarian, fallopian tube, and peritoneal cancer: stages I through IV (1 through 4). -

A Case Report
International Journal of Reproduction, Contraception, Obstetrics and Gynecology Kurude VN et al. Int J Reprod Contracept Obstet Gynecol. 2017 Mar;6(3):1149-1150 www.ijrcog.org pISSN 2320-1770 | eISSN 2320-1789 DOI: http://dx.doi.org/10.18203/2320-1770.ijrcog20170605 Case Report Dysgermgerminoma in a 14 year old girl: a case report V. N. Kurude*, Sukanya Thorat Department of Obstetrics and Gynecology, Grant Medical College and Sir Jamshedjee Jeejeebhoy Group of Hospitals, Mumbai, Maharashtra, India Received: 27 December 2016 Revised: 07 January 2017 Accepted: 31 January 2017 *Correspondence: Dr. V. N. Kurude, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT An accurate diagnosis of ovarian dysgerminoma is crucial as, as its management differs from other ovarian tumours. We report a case of ovarian dyegerminoma in a 14 year old girl who presented with abdominal distention. Examination revealed a huge intra-abdominal mass causing displacement of bowel loops laterally. On ultrasound, a solid heterogeneously hyperechoic lesion of size 18 x 9.4 cm with few cystic and necrotic areas within most likely, left adnexa reaching upto the umbilicus and shows vascularity within both ovaries not seen separately from the lesion. On CT (A+P), a heterogenous hypodense polycystic mass of size 8.5x1.4x16.7 with multiple irregular hypodensities seen in the lower abdomen and pelvis. -
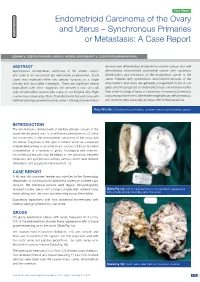
Endometrioid Carcinoma of the Ovary and Uterus – Synchronous Primaries Pathology S Ection Or Metastasis: a Case Report
Case Report ection Endometrioid Carcinoma of the Ovary S and Uterus – Synchronous Primaries Pathology or Metastasis: A Case Report ESWARI V., GEETHA PRAKASH, IRFAN A. ANSARI, BHANUMATHY V., GOMATHI PALVANNANATHAN ABSTRACT showed well differentiated endometrioid ovarian cancer and well Synchronous endometrioid carcinoma of the uterine corpus differentiated endometrioid endometrial cancer with squamous and ovary is an uncommon but well known phenomenon. Such differentiation and metastasis of the endometrial cancer to the cases may represent either two primary tumours or a single cervix. Patients with synchronous endometroid tumours of the primary and associated metastasis. There are significant clinical endometrium and ovary are generally younger,tend to be of low implications with either diagnosis. We present a case of a 48 grade and the prognosis of endometrioid type carcinoma is better year old unmarried women who came to our hospital with Right than other histological types of carcinoma. Immunohistochemistry ovarian mass measuring 13cm. Total abdominal hysterectomy with plays an important role to differentiate single primary with metastasis bilateral salphingoopherectomy was done. Histological examination and dual primaries especially at places with limited resources. Key Words: Synchronous primaries, ovarian cancer, Endometrial cancer INTRODUCTION The simultaneous development of multiple primary cancers in the upper female genital tract is a well known phenomenon. Of these the commonest is the endometrioid carcinoma of the ovary and the uterus. Diagnosis of this type of tumour either as a separate independent primary or as a metastatic tumour is difficult. A careful consideration of a number of gross, histological and immuno- histochemical features may be helpful in the distinction between metastatic and synchronous primary tumours which have different therapeutic and prognostic implications [1, 2]. -

Phytochemicals in Gynecological Cancer Prevention
International Journal of Molecular Sciences Review Phytochemicals in Gynecological Cancer Prevention Marta Wo´zniak 1, Rafał Krajewski 2, Sebastian Makuch 1 and Siddarth Agrawal 1,2,3,* 1 Department of Pathology, Wroclaw Medical University, 50-368 Wroclaw, Poland; [email protected] (M.W.); [email protected] (S.M.) 2 Department and Clinic of Internal Medicine, Occupational Diseases, Hypertension and Clinical Oncology, Wroclaw Medical University, 50-556 Wroclaw, Poland; [email protected] 3 Department of Cancer Prevention and Therapy, Wroclaw Medical University, 50-556 Wroclaw, Poland * Correspondence: [email protected] Abstract: Gynecological cancer confers an enormous burden among women worldwide. Accu- mulating evidence points to the role of phytochemicals in preventing cervical, endometrial, and ovarian cancer. Experimental studies emphasize the chemopreventive and therapeutic potential of plant-derived substances by inhibiting the early stages of carcinogenesis or improving the efficacy of traditional chemotherapeutic agents. Moreover, a number of epidemiological studies have investi- gated associations between a plant-based diet and cancer risk. This literature review summarizes the current knowledge on the phytochemicals with proven antitumor activity, emphasizing their effectiveness and mechanism of action in gynecological cancer. Keywords: phytochemicals; gynecological cancers; anticancer 1. Introduction Citation: Wo´zniak,M.; Krajewski, Currently, there is a dynamic increase in the number of cancer cases around the world. R.; Makuch, S.; Agrawal, S. A total of 18.1 million new cases were reported in 2018, of which nearly 10 million were Phytochemicals in Gynecological fatal [1]. It is estimated that a prolonged human lifespan and limited access to highly Cancer Prevention. Int. J. Mol. -

The Landscape and Therapeutic Implications of Molecular Profiles In
Journal of Clinical Medicine Review The Landscape and Therapeutic Implications of Molecular Profiles in Epithelial Ovarian Cancer Ludivine Dion 1,2,3 , Isis Carton 1,2, Sylvie Jaillard 2,3,4, Krystel Nyangoh Timoh 1,2, Sébastien Henno 5, Hugo Sardain 1, Fabrice Foucher 1, Jean Levêque 1,2, Thibault de la Motte Rouge 6, Susie Brousse 1,2 and Vincent Lavoué 1,2,3,* 1 Service de Chirurgie gynécologique, CHU de Rennes, 35000 Rennes, France; [email protected] (L.D.); [email protected] (I.C.); [email protected] (K.N.T.); [email protected] (H.S.); [email protected] (F.F.); [email protected] (J.L.); [email protected] (S.B.) 2 Faculté de médecine, Université de Rennes 1, 35000 Rennes, France; [email protected] 3 INSERM U 1085, IRSET, Equipe 8, 35000 Rennes, France 4 Service de Cytogénétique, CHU de Rennes, 35000 Rennes, France 5 Service d’anatomo-pathologie, CHU de Rennes, 35000 Rennes, France; [email protected] 6 Service d’oncologie médicale, CRLCC Eugène Marquis, 35000 Rennes, France; [email protected] * Correspondence: [email protected] Received: 24 April 2020; Accepted: 10 July 2020; Published: 15 July 2020 Abstract: Epithelial ovarian cancer (EOC) affects 43,000 women worldwide every year and has a five-year survival rate of 30%. Mainstay treatment is extensive surgery and chemotherapy. Outcomes could be improved by molecular profiling. We conducted a review of the literature to identify relevant publications on molecular and genetic alterations in EOC. -

Primary Ovarian Choriocarcinoma Mimicking Ectopic Pregnancy
Case Report Obstet Gynecol Sci 2014;57(4):330-333 http://dx.doi.org/10.5468/ogs.2014.57.4.330 pISSN 2287-8572 · eISSN 2287-8580 Primary ovarian choriocarcinoma mimicking ectopic pregnancy Eun Jin Heo, Chel Hun Choi, Jung Min Park, Jeong-Won Lee, Duk-Soo Bae, Byoung-Gie Kim Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea Nongestational ovarian choriocarcinoma is an exceedingly rare and highly aggressive tumor. Although early diagnosis and timely initiation of therapy is important, it is difficult in reproductive aged patients because of the frequent elevation of human chorionic gonadotropin. We report a primarily nongestational ovarian choriocarcinoma in a 12-year-old virgin female. Initial diagnosis based on abdominopelvic computed tomography and pelvis magnetic resonance imaging was ectopic pregnancy with hemoperitoneum. A diagnostic laparoscopy of the ovarian tumor revealed choriocarcinoma. Unilateral salpingo-oophorectomy and omental sampling revealed surgical stage of IA. Six courses of adjuvant combination chemotherapy (bleomycin, etoposide, and cisplatin) followed surgery. Keywords: Choriocarcinoma; Laparoscopy; Nongestational; Ovary Introduction nancy, which was finally diagnosed as nongestational ovarian choriocarcinoma. The patient was managed with laparoscopic Ovarian choriocarcinoma is a rare and highly aggressive tumor. ipsilateral salpingo-oophorectomy and surgical staging, fol- It may develop as a metastatic gestational choriocarcinoma lowed by the administration of combination chemotherapy. from uterus or tubal choriocarcinoma, as a primary gestation- al choriocarcinoma associated with ovarian pregnancy, or as a nongestational germ cell tumor differentiating towards the Case report trophoblastic structures [1]. The gestational type is more com- mon than the nongestational type; the estimated incidence of A 12-year-old virgin woman presented with a 20-day history a primary ovarian choriocarcinoma is 1 in 389,000,000. -

A Case of Ovarian Metastasis from Microinvasive Adenosquamous
logy & Ob o st ec e tr n i y c s G Abe et al., Gynecol Obstet (Sunnyvale) 2014, 4:9 Gynecology & Obstetrics DOI; 10.4172/2161-0932.1000248 ISSN: 2161-0932 Case Report Open Access A Case of Ovarian Metastasis from Microinvasive Adenosquamous Carcinoma of the Uterine Cervix Akiko Abe1*, Reiko Furuta2, Yutaka Takazawa2, Eiji Kondo1, Kenji Umayahara1 and Nobuhiro Takeshima1 1Department of Gynecology, Cancer Institute Hospital, Japan 2Division of Pathology, Cancer Institute, Japanese Foundation of Cancer Research, Japan Abstract Background: Ovarian metastasis is rare in cases of early-stage uterine cervical cancer. For the patients with stage 1b cervical cancer, the incidences of ovarian metastasis were 0.22% of squamous cell carcinoma and 3.72% of adenocarcinoma. The safety of ovarian preservation is controversial for young women, although these women may find it important to preserve fertility. Case: A 36-year-old Japanese woman underwent a loop electrosurgical excision procedure for cervical adenosquamous carcinoma with invasion of 0.8 mm in depth and 1 mm in horizontal extent. She wished to preserve her fertility and was therefore followed up without additional treatments. Thirty months after the loop electrosurgical excision procedure, she had 10 cm-diameter ovarian tumors and underwent hysterectomy, bilateral salpingo- oophorectomy, appendectomy. This ovarian tumor was revealed to metastasis from cervical carcinoma. Conclusion: To our knowledge, this is first reported case of ovarian metastasis with microinvasive adenosquamous cell carcinoma. The pathological characteristics are important for prognosis: frequent small foci of invasion and high atypia. Keywords: Cervical cancer; Metastasis; Ovarian recurrence; HPV She underwent hysterectomy, bilateral salpingo-oophorectomy, appendectomy because of the mucinous feature of the tumor, and low Case and Method anterior resection of the rectum because of the rectal serosal tumor Ovarian metastasis rarely occurs in cases of early stage uterine invasion. -

Ovarian Cystadenocarcinoma with an Osteosarcoma Component
Global Journal of Anesthesia & Pain Medicine DOI: 10.32474/GJAPM.2021.04.000190 ISSN: 2644-1403 Case Report Ovarian Cystadenocarcinoma With an Osteosarcoma Component Sakhri esp Boulhart S* Head of medical oncology department Laghouat, Algeria *Corresponding author: Sakhri esp Boulhart S, Head of medical oncology department Laghouat, Algeria Received: June 19, 2021 Published: July 2, 2021 Abstract Ovarian cystadenocarcinoma with an osteosarcoma component, is a very rare tumor. We aimed to describe a case of an osteosarcoma arising in an ovarian squamous cell carcinoma. Introduction carboplatin as adjuvant therapy. The patient was free of disease at Ovarian, cystadenocarcinoma with an osteosarcoma com- the 7-month follow-up consultation. ponent (OCS) also known as malignant mixed müllerian tumor (MMMT), is a very rare gynecological malignancy accounting for Discussion 1-3% of ovarian malignancies [1]. OCS is a mixed tumor composed of carcinomatous and sarcomatous components. The sarcomatous OCS is an extremely rare tumor among ovarian cancers, with component may be either homologous, including endometrial stro- a frequency of occurrence of 1–3% [1]. Carcinosarcomas of the female genital tract are often found after menopause at a median We report a case of ovarian cystadenocarcinoma with an osteosar- age of 60 to 70 years old. OCS has a worse survival rate than mal sarcoma, fibrosarcoma and leiomyosarcoma, or heterologous. coma component. high-grade ovarian cancer at the same FIGO stage, with a median overall survival ranging from 7 to 27months [1]. Histologically, Case Presentation OCS contains both carcinomatous (malignant epithelial) and sarcomatous (mesenchymal) components. The sarcomatous A 63-year-old woman presented a tumor in the abdomen component may consist of homologous tissue that are native to the ovary or heterologous tissue not native to the ovary.