Sulfuric Acid Interim AEGL Document
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sodium Sulfate CAS N°: 7757-82-6
OECD SIDS SODIUM SULFATE FOREWORD INTRODUCTION Sodium sulfate CAS N°: 7757-82-6 UNEP PUBLICATIONS 1 OECD SIDS SODIUM SULFATE SIDS Initial Assessment Report For SIAM 20 Paris, France, 19 – 22 April 2005 1. Chemical Name: Sodium sulfate 2. CAS Number: 7757-82-6 3. Sponsor Country: Slovak Republic Contact Point: Centre for Chemical Substances and Preparations, Bratislava Contact Person: Peter Rusnak, Ph.D. Director Co-sponsor Country: Czech Republic Contact Point: Ministry of Environment Contact Person: Karel Bláha, Ph.D. Director Department of Environmental Risks Prague 4. Shared Partnership with: Sodium Sulfate Producers Association (SSPA)∗ and TOSOH 5. Roles/Responsibilities of the Partners: • Name of industry sponsor Sodium Sulfate Producers Association (SSPA) /consortium • Process used Documents were drafted by the consortium, then peer reviewed by sponsor countries experts 6. Sponsorship History • How was the chemical or Nominated by ICCA in the framework of the ICCA HPV category brought into the program OECD HPV Chemicals Programme? 7. Review Process Prior to Two drafts were reviewed by the Slovakian/Czech authorities; third the SIAM: draft subject to review by OECD membership 8. Quality check process: Data was reviewed against the OECD criteria as described in the SIDS manual. These criteria were used to select data for extraction into the SIDS dossier. Original data was sought wherever possible. Originally reported work was deemed reliable if sufficient information was reported (according to the manual) to judge it robust. Reviews were only judged reliable if reported 2 UNEP PUBLICATIONS OECD SIDS SODIUM SULFATE by reputable organisations/authorities or if partners had been directly involved in their production 9. -
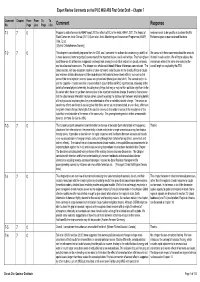
Comment Response 7-1 7 0 Propose to Add References to AMAP Report 2012 on Effects of BC in the Arctic
Expert Review Comments on the IPCC WGI AR5 First Order Draft -- Chapter 7 Comment Chapter From From To To No Page Line Page Line Comment Response 7-1 7 0 Propose to add references to AMAP report 2012 on effects of BC in the Arctic. AMAP, 2011. The Impact of reviewer needs to be specific as to where this fits. Black Carbon on Arctic Climate (2011). Quinn et al. Arctic Monitoring and Assessment Programme (AMAP), Preference goes to peer-reviewed literature. Oslo. 72 pp. [Øyvind Christophersen, Norway] 7-2 7 0 This chapter is considerably improved from the ZOD, and I commend the authors for constructing a draft that We concur with these comments about the amounts is more balanced, better organized, covers most of the important issues, and is well written. That having been of detail in each section. We will try to address the said, there are still differences in approach and emphasis among the individual sections on clouds, aerosols, imbalances while at the same time reducing the and cloud-aerosol interactions. The chapter as a whole would benefit if these differences were reduced. The overall length as required by the TSU. clouds section, with one exception noted in a future comment, really focuses on the climatic effects of clouds and eschews detailed discussions of the cloud physics that leads to these climate effects, so much so that some of the more important science issues are ignored and others given short shrift. The aerosol section is just the opposite - it reads more like a review article in a journal than an IPCC report section, reviewing all the details of aerosol physics/chemistry, including many things that may or may not turn out to be significant in the future but which have not yet been demonstrated to be important to climate change. -

Preparation of Soil Nutrient Amendment Using White Mud Produced in Ammonia-Soda Process and Its Environmental Assessment
Preparation of soil nutrient amendment using white mud produced in ammonia-soda process and its environmental assessment SHI Lin(石 林), LUO Han-jin(罗汉金) College of Environmental Science and Engineering, South China University of Technology, Guangzhou 510006, China Received 15 July 2009; accepted 7 September 2009 Abstract: A novel method to prepare soil nutrient amendment by calcining a mixture of white mud and potassium feldspar and its for white mud to 30׃environmental assessment were investigated. Under the optimal conditions of a blending mass ratio of 70 potassium feldspar, a calcination temperature of 1 000 ℃, a calcination time of 1.5 h and spherulitic diameter of 2.0 cm, the calcined product, as a soil nutrient amendment, could be prepared with the following nutrient composition (mass fraction): K2O 4.16%, CaO 2− − 23.43%, MgO 5.04%, SiO2 22.92%, SO4 3.71%, and Cl 3.87% in 0.1 mol/L citric acid solution. The concentrations of heavy metals in the calcined product and the emission concentrations of harmful gases from a mixture of white mud and potassium feldspar during calcination process could qualify the National Standards without causing secondary environmental pollution. Key words: white mud; potassium feldspar; soil nutrient amendment; environmental assessment; preparation white mud, in combination with the above-mentioned 1 Introduction factors, makes it become a serious obstruction for application in the building and cement industry. So far, Distilled waste sludge containing 50%−60% water, the common treatment of white mud is still to discard it commonly known as “white mud”, is discharged from into waterway or dispose of it in landfills. -

Designing an Adaptive Building Envelope for Warm-Humid Climate Using Bamboo Veneer As a Hygroscopically Active Material
The Pennsylvania State University The Graduate School RESPONSIVE SKIN: DESIGNING AN ADAPTIVE BUILDING ENVELOPE FOR WARM-HUMID CLIMATE USING BAMBOO VENEER AS A HYGROSCOPICALLY ACTIVE MATERIAL A Thesis in Architecture by Manal Anis 2019 Manal Anis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science May 2019 ii The thesis of Manal Anis was reviewed and approved* by the following: Marcus Shaffer Associate Professor of Architecture Thesis Advisor Ute Poerschke Professor of Architecture Interim Head of the Department of Architecture Benay Gursoy Toykoc Assistant Professor of Architecture Rebecca Henn Associate Professor of Architecture Director of Graduate Studies of the Department of Architecture *Signatures are on file in the Graduate School iii abstract Architectural facades that are able to adapt themselves in response to changing climatic conditions have typically been identified with having high-tech complex automated mechanisms, using electronic sensors and actuators. The low-tech and no- tech passive strategies of adaptive façade design based on material responsiveness are still in their infancy. Passive strategies minimize energy and material use while maintaining occupant comfort. This is precisely why such methods require a greater emphasis today as we investigate deeper into the realms of Responsive Architecture. Materials such as bamboo and wood undergo a natural, biological reaction to environmental changes, and, therefore, offer an opportunity for non-mechanical adaption. Bamboo, due to its hygroscopic nature, undergoes constant expansion and contraction with changing levels of atmospheric humidity. From a crafting and constructing perspective, this spontaneous dimensional change – material instability - was seen as an inherent drawback of working with bamboo, with attempts being made to control, mitigate, or counteract the change. -

Hygroscopy-Induced Nanoparticle Reshuffling in Ionic-Gold-Residue
Showcasing research from the Department of Chemistry As featured in: Education, Seoul National University, Seoul, South Korea. Hygroscopy-induced nanoparticle reshuffl ing in ionic-gold-residue-stabilized gold suprananoparticles Ionic precursors play a role in the generation of nanoparticles and infl uence their properties. The excess gold ions facilitate the formation of ultra-small gold nanoparticles and the further assembly of these small nanoparticles into suprananoparticles. The hygroscopic Au(III) residues in the suprananoparticles absorb moisture to form a micro-water pool and, subsequently, the nanoparticles are able to reshuffl e to larger nanoparticles, which is used to detect the water content in organic solvents. Our results indicate that nanoparticle precursors may introduce See Junhua Yu et al., additional characteristics and signifi cantly change the properties Nanoscale Adv., 2019, 1, 1331. of nanostructures. rsc.li/nanoscale-advances Registered charity number: 207890 Nanoscale Advances View Article Online PAPER View Journal | View Issue Hygroscopy-induced nanoparticle reshuffling in ionic-gold-residue-stabilized gold Cite this: Nanoscale Adv.,2019,1, 1331 suprananoparticles† Sungmoon Choi, ‡ Minyoung Lim,‡ Yanlu Zhao and Junhua Yu * Polyethyleneimine (PEI)-stabilized gold nanoparticles were used as a model to understand the roles of ionic precursors in the formation of nanoparticles and the impact of their presence on the nanoparticle properties. The low availability of elemental gold and the stabilization of the just-generated gold nanoparticles by the excess gold ions contributed to the production of ultra-small nearly neutral gold nanoparticles, resulting in properties significantly different from those prepared by conventional methods. The cross-linking between gold ions/PEI/nanoparticles further led to the assembly of these small gold nanoparticles into suprananoparticles that were stable in water. -

Development of Graphene Oxide Based Aerogels
DEVELOPMENT OF GRAPHENE OXIDE BASED AEROGELS A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF NATURAL AND APPLIED SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY ÖZNUR DOĞAN IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN CHEMICAL ENGINEERING AUGUST 2017 Approval of thesis: DEVELOPMENT OF GRAPHENE OXIDE BASED AEROGELS submitted by ÖZNUR DOĞAN in partial fulfillment of the requirements for the degree of Master of Science in Chemical Engineering Department, Middle East Technical University by, Prof. Dr. Gülbin Dural Ünver Dean, Graduate School of Natural and Applied Sciences _______________ Prof. Dr. Halil Kalıpçılar Head of Department, Chemical Engineering _______________ Asst. Prof. Dr. Erhan Bat Supervisor, Chemical Engineering Dept., METU _______________ Examining Committee Members: Prof. Dr. Levent Yılmaz _______________ Chemical Engineering Dept., METU Asst. Prof. Dr. Erhan Bat _______________ Chemical Engineering Dept., METU Assoc. Prof. Dr. Bora Maviş _______________ Mechanical Engineering Dept., Hacettepe University Asst. Prof. Dr. Emre Büküşoğlu _______________ Chemical Engineering Dept., METU Asst. Prof. Dr. Bahar İpek _______________ Chemical Engineering Dept., METU Date: 18.08.2017 I hereby declare that all information in this document has been obtained and presented in accordance with academic rules and ethical conduct. I also declare that, as required by these rules and conduct, I have fully cited and referenced all material and results that are not original to this work. Name, Last Name: Öznur Doğan Signature: iv ABSTRACT DEVELOPMENT OF GRAPHENE OXIDE BASED AEROGELS Doğan, Öznur M.Sc., Department of Chemical Engineering Supervisor: Asst. Prof. Dr. Erhan Bat August 2017, 101 pages Owing to their large surface area, high hydrophobicity and porous structure, graphene oxide based aerogels have been proposed as feasible and economic solutions for the increasing water pollution caused by crude oils, petroleum products and toxic organic solvents. -

Kinetics of Dissolution and Recrystallization of Sodium Chloride at Controlled Relative Humidity†
Kinetics of Dissolution and Recrystallization of Sodium Chloride at Controlled Relative Humidity† Marina Langlet1*, Frederic Nadaud2, Mohamed Benali1, Isabelle Pezron1, Khashayar Saleh1, Pierre Guigon1, Léa Metlas-Komunjer1 UTC/ESCOM, Équipe d’Accueil “Transformations Intégrées de la Matière Renouvelable” (EA 4297)1 UTC/ESCOM, Service d’Analyses Physico-Chimique2, Abstract Both producers and users of divided solids regularly face the problem of caking after periods of storage and/or transport. Particle agglomeration depends not only on powder water content, tem- perature and applied pressure, but also on the interactions between the solid substance and water molecules present in the atmosphere, i.e. on relative humidity (RH) at which the product is stored. Ambient humidity plays an important role in most events leading to caking: capillary condensation of water at contact points between particles, subsequent dissolution of a solid and formation of a saturated solution eventually followed by precipitation of the solid during the evaporation of water. Here, we focus on the kinetics of dissolution followed by evapo-recrystallization of a hygroscopic so- dium chloride powder under controlled temperature and RH, with the aim of anticipating caking by predicting rates of water uptake and loss under industrial conditions. Precise measurements of water uptake show that the rate of dissolution is proportional to the difference between the imposed RH and deliquescence RH, and follows a model based on the kinetic theory of gases. Evaporation seems to be governed by more complex phenomena related to the mechanism of crystal growth from a supersatu- rated salt solution. Keywords: Sodium chloride, hygroscopy, Knudsen law, vapor sorption, caking humidity, DRH, of 75% at 25℃. -

Final Report CCQM-P32
Eidgenössische Materialprüfungs- und Forschungsanstalt Lerchenfeldstrasse 5 Laboratoire fédéral d'essai des matériaux et de recherche Postfach 755 Laboratorio federale di prova dei materiali e di ricerca CH-9014 St. Gallen Institut federal da controlla da material e da retschertgas Tel. +41-71-274 74 74 Swiss Federal Laboratories for Materials Testing and Research Fax +41-71-274 74 99 International Intercomparison Pilot Study CCQM-P32 on Anion Calibration Solutions Final Report 2003 Michael Weber Jürg Wüthrich CCQM-P32 Pilot Study Anion Calibration Solution 1. Abstract In CCQM-P32 pilot study two anion calibration solutions of chloride and phosphate were investigated. The contents of both solutions were about 1 g/kg relative to the anion mass. For the chloride comparison 11 participants provided 16 results by the following analytical techniques: coulometry (7), titrimetry (5) and ion chromatography (4). The phosphate amount content was determined by 9 NMIs and 11 results were submitted. For phosphate ion chromatography was the most used technique (4) followed by titrimetry (2), ICP-OES (2), gravimetry (1) and ion-exchange-coulometry (1). All results were found within the range of ± 0.5% with respect to the gravimetric value. The variability (RSD) of the results is 0.13% for the chloride solution and 0.26% for the phosphate solution. Compared to the results of CCQM-K8 (monoelemental solutions, average RSD 0.25%) these results are quite similar. No significant deviation between the mean of all measured values and the gravimetric value were found in both samples. A key comparison is planned for 2003. 2. Rationale of this Study Aqueous solutions of anions are widely used for the calibration in analytical chemistry. -

Study of the Hygroscopic Deterioration of Ammonium Perchlorate / Magnesium Mixture
Sci. Tech. Energetic Materials, Vol.79,No.1,2018 15 Research 4 paper 1 9 Study of the hygroscopic deterioration of ammonium perchlorate/magnesium mixture Yo su k e N ishiwaki*,Takehiro Matsunaga**,andMieko Kumasaki*† *Graduate School of Environment and lnformation Sciences, Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku, Yokohama-shi, Kanagawa, 240-8501 JAPAN Phone: +81-45-339-3994 †Corresponding author: [email protected] **National Institute of Advanced Industrial Science and Technology, 1-1-1 Higashi, Tsukuba-shi, Ibaraki, 305-8565 JAPAN Received: December 21, 2016 Accepted: February 6, 2018 Abstract AStudy of hygroscopic deterioration is necessary because it can improve the safety and quality of pyrotechnics. For effective prevention of hygroscopic deterioration, this paper shows the deterioration mechanism of ammonium perchlorate (AP)/magnesium (Mg) mixture under the presence of moisture. The mixture has been used as Tenmetsuzai (Strobe composition in Japanese) and Mg has been known for its high hygroscopy. In this paper, the hygroscopic deterioration of strobe composition (AP/Mgmixture)wasmeasured by aging test which we were developed, and the deterioration products were analyzed with X-raydiffraction system and scanning electron microscope. The experiments identified two deterioration products : magnesium hydroxide and magnesium perchlorate. The influence of storage temperature and humidity to the rate of deterioration were investigated, and it revealed that the relative humidity of atmosphere has a significant impact. AP/Mg showeddrastic change at a point, and the point was considered to be Critical Relative Humidity. The rate equation which called Jander’s equation was applied to the deterioration behavior obtained in the experiments, and its coefficientswereobtained which enable to predict the degree of deterioration as function of time. -

Manual for Refrigeration Servicing Technicians
Manual for Refrigeration Servicing Introduction Technicians Welcome to the Manual for Refrigeration Servicing Technicians. It is an e-book for people who are involved in training and organization of service and maintenance of refrigeration and air- conditioning (RAC) systems. It is aimed at people who are: • Service and maintenance technicians • Private company service/maintenance managers • Private company managers involved in developing their Why you need this manual 4 service and maintenance policy Private company technicians trainers When to use this manual 4 • • Educational establishment RAC trainers and course How to use this manual 4 developers • National Ozone Units (NOUs) responsible for servicing and maintenance regulations and programmes related to the Montreal Protocol. Why you need this Manual Over recent years, attention on the issue of ozone depletion has The manual is written for those who have a relatively comprehensive remained focused on the obligatory phasing out of ozone depleting level of knowledge and understanding of RAC systems and substances (ODS). At the same time, awareness of climate change associated technology. The material within this manual may be Introduction has increased, along with the development of national and regional used for the purpose of developing training resources or parts greenhouse gas (GHG) emissions reduction targets. In order to of training courses, as well as general guidance and information achieve reduction in emissions of both ODSs and GHGs, attention for technicians on issues that are closely related to the use and has to be paid to activities at a micro-level. This includes reducing application of alternative refrigerants. Most training courses are leakage rates, improving energy-efficiency and preventing other likely to cover a range of topics associated with RAC systems, and environmental impacts, by directing the activities of individuals, and as such, the material within this manual may contribute towards influencing the design and maintenance of equipment. -

A Simple Model for the Hygroscopy of Sulphuric Acid
A Simple Model for the Hygroscopy of Sulphuric Acid , Kristian B. Kiradjiev,∗ y Vladimiros Nikolakis,z Ian M. Griffiths,y Uwe Beuscher,z Vasudevan Venkateshwaran,z and Christopher J. W. Brewardy Mathematical Institute, University of Oxford, Woodstock Rd, OX2 6GG, Oxford, UK y W. L. Gore and Associates, Inc., 100 Airport Rd, Elkton, MD 21921, USA z E-mail: [email protected] Abstract Sulphuric acid is a highly hygroscopic substance, increasing its volume by absorbing water from high relative-humidity environment. In this paper, we present a mathemati- cal model that describes the hygroscopy of a uniform layer of sulphuric acid with a given initial concentration and relative humidity of the surrounding gaseous environment. We assume that water is absorbed across the gas–liquid interface at a rate proportional to the difference in concentration from the equilibrium value. Our numerical results com- pare well with asymptotic predictions for small Sherwood number, where we derive an explicit solution. The theory agrees well with experimental data, which supports the validity of the model, and we are able to use the model to determine the rate of absorption, which cannot be found by a direct experimental measurement. 1 Introduction Sulphuric acid is used in a wide range of industrial applications, including as a catalyst in alkylation reactions, in petroleum refining, in the manufacture of detergents, and as an 1 important component in lead-acid batteries1. Sulphuric acid can be produced as a by- product, for example, when removing sulphur dioxide from flue gas. Its properties have been extensively studied and established empirically2–4. -

A High Temperature Capacitive Humidity Sensor Based on Mesoporous Silica
Sensors 2011, 11, 3135-3144; doi:10.3390/s110303135 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Article A High Temperature Capacitive Humidity Sensor Based on Mesoporous Silica Thorsten Wagner 1,*, Sören Krotzky 2, Alexander Weiß 3, Tilman Sauerwald 3, Claus-Dieter Kohl 3, Jan Roggenbuck 4 and Michael Tiemann 1 1 Department of Chemistry, Faculty of Science, University of Paderborn, Warburger Str. 100, D-33098 Paderborn, Germany; E-Mail: [email protected] 2 Max-Planck-Institute for Solid State Research, Nanoscale Science Department, Heisenbergstr. 1, D-70569 Stuttgart, Germany; E-Mail: [email protected] 3 Institute for Applied Physics, Justus Liebig University, Heinrich-Buff-Ring 16, D-35390 Giessen, Germany; E-Mails: [email protected] (A.W.); [email protected] (T.S.); [email protected] (C.-D.K.) 4 Institute of Inorganic and Analytical Chemistry, Justus Liebig University, Heinrich-Buff-Ring 16, D-35390 Giessen, Germany * Author to whom correspondence should be addressed; E-Mail: [email protected]. Received: 30 January 2011; in revised form: 20 February 2011 / Accepted: 25 February 2011 / Published: 14 March 2011 Abstract: Capacitive sensors are the most commonly used devices for the detection of humidity because they are inexpensive and the detection mechanism is very specific for humidity. However, especially for industrial processes, there is a lack of dielectrics that are stable at high temperature (>200 °C) and under harsh conditions. We present a capacitive sensor based on mesoporous silica as the dielectric in a simple sensor design based on pressed silica pellets.