Temporomandibular Disorders and Oral Features in Systemic Lupus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Oral Diagnosis: the Clinician's Guide
Wright An imprint of Elsevier Science Limited Robert Stevenson House, 1-3 Baxter's Place, Leith Walk, Edinburgh EH I 3AF First published :WOO Reprinted 2002. 238 7X69. fax: (+ 1) 215 238 2239, e-mail: [email protected]. You may also complete your request on-line via the Elsevier Science homepage (http://www.elsevier.com). by selecting'Customer Support' and then 'Obtaining Permissions·. British Library Cataloguing in Publication Data A catalogue record for this book is available from the British Library Library of Congress Cataloging in Publication Data A catalog record for this book is available from the Library of Congress ISBN 0 7236 1040 I _ your source for books. journals and multimedia in the health sciences www.elsevierhealth.com Composition by Scribe Design, Gillingham, Kent Printed and bound in China Contents Preface vii Acknowledgements ix 1 The challenge of diagnosis 1 2 The history 4 3 Examination 11 4 Diagnostic tests 33 5 Pain of dental origin 71 6 Pain of non-dental origin 99 7 Trauma 124 8 Infection 140 9 Cysts 160 10 Ulcers 185 11 White patches 210 12 Bumps, lumps and swellings 226 13 Oral changes in systemic disease 263 14 Oral consequences of medication 290 Index 299 Preface The foundation of any form of successful treatment is accurate diagnosis. Though scientifically based, dentistry is also an art. This is evident in the provision of operative dental care and also in the diagnosis of oral and dental diseases. While diagnostic skills will be developed and enhanced by experience, it is essential that every prospective dentist is taught how to develop a structured and comprehensive approach to oral diagnosis. -
Median Rhomboid Glossitis
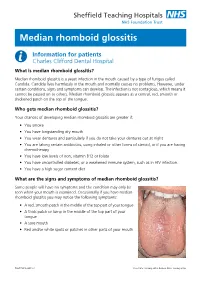
Median rhomboid glossitis Information for patients Charles Clifford Dental Hospital What is median rhomboid glossitis? Median rhomboid glossitis is a yeast infection in the mouth caused by a type of fungus called Candida. Candida lives harmlessly in the mouth and normally causes no problems. However, under certain conditions, signs and symptoms can develop. The infection is not contagious, which means it cannot be passed on to others. Median rhomboid glossitis appears as a central, red, smooth or thickened patch on the top of the tongue. Who gets median rhomboid glossitis? Your chances of developing median rhomboid glossitis are greater if: • You smoke • You have longstanding dry mouth • You wear dentures and particularly if you do not take your dentures out at night • You are taking certain antibiotics, using inhaled or other forms of steroid, or if you are having chemotherapy • You have low levels of iron, vitamin B12 or folate • You have uncontrolled diabetes, or a weakened immune system, such as in HIV infection. • You have a high sugar content diet What are the signs and symptoms of median rhomboid glossitis? Some people will have no symptoms and the condition may only be seen when your mouth is examined. Occasionally if you have median rhomboid glossitis you may notice the following symptoms: • A red, smooth patch in the middle of the top part of your tongue • A thick patch or lump in the middle of the top part of your tongue • A sore mouth • Red and/or white spots or patches in other parts of your mouth PD6779-PIL2645 v4 Issue Date: January 2019. -

Zeroing in on the Cause of Your Patient's Facial Pain
Feras Ghazal, DDS; Mohammed Ahmad, Zeroing in on the cause MD; Hussein Elrawy, DDS; Tamer Said, MD Department of Oral Health of your patient's facial pain (Drs. Ghazal and Elrawy) and Department of Family Medicine/Geriatrics (Drs. Ahmad and Said), The overlapping characteristics of facial pain can make it MetroHealth Medical Center, Cleveland, Ohio difficult to pinpoint the cause. This article, with a handy at-a-glance table, can help. [email protected] The authors reported no potential conflict of interest relevant to this article. acial pain is a common complaint: Up to 22% of adults PracticE in the United States experience orofacial pain during recommendationS F any 6-month period.1 Yet this type of pain can be dif- › Advise patients who have a ficult to diagnose due to the many structures of the face and temporomandibular mouth, pain referral patterns, and insufficient diagnostic tools. disorder that in addition to Specifically, extraoral facial pain can be the result of tem- taking their medication as poromandibular disorders, neuropathic disorders, vascular prescribed, they should limit disorders, or atypical causes, whereas facial pain stemming activities that require moving their jaw, modify their diet, from inside the mouth can have a dental or nondental cause and minimize stress; they (FIGURE). Overlapping characteristics can make it difficult to may require physical therapy distinguish these disorders. To help you to better diagnose and and therapeutic exercises. C manage facial pain, we describe the most common causes and underlying pathological processes. › Consider prescribing a tricyclic antidepressant for patients with persistent idiopathic facial pain. C Extraoral facial pain Extraoral pain refers to the pain that occurs on the face out- 2-15 Strength of recommendation (SoR) side of the oral cavity. -

Pediatric Oral Pathology. Soft Tissue and Periodontal Conditions
PEDIATRIC ORAL HEALTH 0031-3955100 $15.00 + .OO PEDIATRIC ORAL PATHOLOGY Soft Tissue and Periodontal Conditions Jayne E. Delaney, DDS, MSD, and Martha Ann Keels, DDS, PhD Parents often are concerned with “lumps and bumps” that appear in the mouths of children. Pediatricians should be able to distinguish the normal clinical appearance of the intraoral tissues in children from gingivitis, periodontal abnormalities, and oral lesions. Recognizing early primary tooth mobility or early primary tooth loss is critical because these dental findings may be indicative of a severe underlying medical illness. Diagnostic criteria and .treatment recommendations are reviewed for many commonly encountered oral conditions. INTRAORAL SOFT-TISSUE ABNORMALITIES Congenital Lesions Ankyloglossia Ankyloglossia, or “tongue-tied,” is a common congenital condition characterized by an abnormally short lingual frenum and the inability to extend the tongue. The frenum may lengthen with growth to produce normal function. If the extent of the ankyloglossia is severe, speech may be affected, mandating speech therapy or surgical correction. If a child is able to extend his or her tongue sufficiently far to moisten the lower lip, then a frenectomy usually is not indicated (Fig. 1). From Private Practice, Waldorf, Maryland (JED); and Department of Pediatrics, Division of Pediatric Dentistry, Duke Children’s Hospital, Duke University Medical Center, Durham, North Carolina (MAK) ~~ ~ ~ ~ ~ ~ ~ PEDIATRIC CLINICS OF NORTH AMERICA VOLUME 47 * NUMBER 5 OCTOBER 2000 1125 1126 DELANEY & KEELS Figure 1. A, Short lingual frenum in a 4-year-old child. B, Child demonstrating the ability to lick his lower lip. Developmental Lesions Geographic Tongue Benign migratory glossitis, or geographic tongue, is a common finding during routine clinical examination of children. -

The Treatment of Herpes Labialis with a Diode Laser (970 Nm) — a Field Study
I clinical article The treatment of herpes labialis with a diode laser (970 nm)—a field study DrSimoneSuppelt AbstrAct Herpes labialis is an infection caused by the herpes simplex virus HSV 1 and, less frequently, HSV 2. In dental prac - tices the diode laser is mainly used in periodontology, endodontics and minimally invasive surgery. Many of those affected by herpes are unaware that laser treatment can successfully alleviate their symptoms. In this field study, 11 patients who suffer from acute herpes were treated with a 970 nm diode laser. The areas which the patients described as being affected by herpes were irradiated at a distance of 1 –3 mm (2.0 W, 10 Hz, 50 % duty cycle, 320 µm optical fiber). Several patients felt the symptoms subside during the treatment. For the majority of patients, the symptoms did not occur again after treatment. All of the patients were satisfied with the treatment. Laser treatment of herpes labialis using a 970 nm diode laser is an effective way for me to help my patients both quickly and simply. Keywords Diode laser, 970 nm, herpes labialis, HSV Introduction An outbreak of herpes labialis can be accompanied by var - ious symptoms. As a rule, in the early stages such symptoms With a wavelength of 970 nm and a maximum output of include dry lips and a tingling/itching sensation. In subsequent 7W cw, the SIROLaser Advance dental diode laser has a wide stages, swelling and a feeling of tightness occur which can range of indications. In my practice, the laser is mainly used rapidly be accompanied by a sensation of burning or other in periodontology and endodontics to reduce germs in pock - sense of pain. -

Alcohol Use and Oral Health Fact Sheet for PROVIDERS OCTOBER 2017
Alcohol Use and Oral Health Fact Sheet FOR PROVIDERS OCTOBER 2017 The Challenge… Glossitis – tongue inflammation Patients who drink alcohol regularly may experience specific problems related to their oral health and hygiene. Angular cheilitis – corners of the mouth chronically inflamed and cracked What you need to know… Candida – yeast infection • Patients who drink high amounts of alcohol daily may brush Oral Ulceration – painful round or oval less effectively than those who don’t drink alcohol, despite sores reporting similar brushing frequency. Also, impaired motor Acute Necrotizing activity can affect their ability to perform basic dental hygiene adequately.1 Ulcerative Gingivitis – infection of the gums that causes ulcers, swelling, and • Alcohol is also the most common cause of sialadenosis dead tissue in the mouth of the parotid gland. This condition causes swelling of the parotid gland and decreased secretion of saliva.2 Ways You Can Help… • Poor nutrient intake and absorption combined with decreased salivary excretion frequently can lead to glossitis, Recommend: angular cheilitis, candida infection, oral ulceration, and acute • Brushing thoroughly two times daily with a necrotizing ulcerative gingivitis (ANUG).2 fluoridated toothpaste. • A decreased immune response combined with a nutritionally • Rinse mouth with non-alcoholic mouth rinse. poor diet, poor oral hygiene, decreased salivary flow, and a • Have an oral examination and cleaning by a high incidence of smoking among these patients, provides dental professional at least two times per year. an environment conducive to rapid progression of periodontal • Regular oral exams that include a periodontal disease, dental caries and increased risk of oral thoracic evaluation and oral cancer screenings to detect cancers.2 any signs of suspicious lesions.3 • High consumption of alcohol may damage the liver and bone marrow resulting in excessive bleeding during dental treatment. -

Therapeutic Management of Patients with Class III Skeletal Malocclusion
Szpyt Justyna, Gębska Magdalena. Therapeutic management of patients with class III skeletal malocclusion. Mandibular prognathism, maxillary retrognathism – a case report. Journal of Education, Health and Sport. 2019;9(5):20-31. eISSN 2391-8306. DOI http://dx.doi.org/10.5281/zenodo.2656446 http://ojs.ukw.edu.pl/index.php/johs/article/view/6872 https://pbn.nauka.gov.pl/sedno-webapp/works/912455 The journal has had 7 points in Ministry of Science and Higher Education parametric evaluation. Part B item 1223 (26/01/2017). 1223 Journal of Education, Health and Sport eISSN 2391-8306 7 © The Authors 2019; This article is published with open access at Licensee Open Journal Systems of Kazimierz Wielki University in Bydgoszcz, Poland Open Access. This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author (s) and source are credited. This is an open access article licensed under the terms of the Creative Commons Attribution Non commercial license Share alike. (http://creativecommons.org/licenses/by-nc-sa/4.0/) which permits unrestricted, non commercial use, distribution and reproduction in any medium, provided the work is properly cited. The authors declare that there is no conflict of interests regarding the publication of this paper. Received: 15.04.2019. Revised: 25.04.2019. Accepted: 01.05.2019. Therapeutic management of patients with class III skeletal malocclusion. Mandibular prognathism, maxillary retrognathism – a case report Justyna Szpyt1, Magdalena Gębska2 1. Physiotherapy student, Faculty of Health Sciences, Pomeranian Medical University in Szczecin. -
Pan Imaging News V3 I4
Volume 4, Issue 4 US $6.00 Editor: Allan G. Farman, BDS, PhD (odont.), Assessing Growth and Development with Panoramic DSc (odont.), Diplomate of the Radiographs and Cephalometric Attachments: A critical American Board of Oral and Maxillofacial Radiology, Professor of tool for dental diagnosis and treatment planning Radiology and Imaging Sciences, Department of Surgical and Hospital By Dr. Allan G. Farman Dentistry, The University of It is recommended that radio- indicator of age is that the three Louisville School of Dentistry, graphs be made periodically including permanent molar teeth in each quad- Louisville, KY. both during the mixed dentition (8-9 rant erupt approximately at six-year year old) and adolescence (12-14 year intervals. The first permanent molar Featured Article: old) to evaluate growth and develop- erupts around 6 years, the second Assessing Growth and Development ment, and to look for asymptomatic permanent molar around 12 years, and with Panoramic Radiographs and dental disease [1-3]. Substantial the third molars around 18 years. Root Cephalometric Attachments: A critical differences in the assessed biological formation for permanent teeth is tool for dental diagnosis and and the known chronological age can completed roughly three years following treatment planning be indicators of a variety of inherited eruption. The first major attempt at and congenital conditions. Further, local developing a chronology for human In The Recent Literature: failure in dental eruption within the tooth development was that of Logan normal time range can be evidence of and Kronfeld (1933) and with minor Implantology dental impaction and possibly of a modification is still usable as a rough pathological process such as a hamar- and ready guide. -

Cardiovascular Drugs-Induced Oral Toxicities: a Murky Area to Be Revisited and Illuminated
Pharmacological Research 102 (2015) 81–89 Contents lists available at ScienceDirect Pharmacological Research j ournal homepage: www.elsevier.com/locate/yphrs Review Cardiovascular drugs-induced oral toxicities: A murky area to be revisited and illuminated a, b b Pitchai Balakumar ∗, Muthu Kavitha , Suresh Nanditha a Pharmacology Unit, Faculty of Pharmacy, AIMST University, Semeling, 08100 Bedong, Malaysia b Faculty of Dentistry, AIMST University, 08100 Bedong, Malaysia a r t i c l e i n f o a b s t r a c t Article history: Oral health is an imperative part of overall human health. Oral disorders are often unreported, but are Received 20 July 2015 highly troublesome to human health in a long-standing situation. A strong association exists between Received in revised form 22 August 2015 cardiovascular drugs and oral adverse effects. Indeed, several cardiovascular drugs employed clinically Accepted 8 September 2015 have been reported to cause oral adverse effects such as xerostomia, oral lichen planus, angioedema, Available online 25 September 2015 aphthae, dysgeusia, gingival enlargement, scalded mouth syndrome, cheilitis, glossitis and so forth. Oral complications might in turn worsen the cardiovascular disease condition as some reports suggest an Keywords: adverse correlation between periodontal oral disease pathogenesis and cardiovascular disease. These are Cardiovascular drugs certainly important to be understood for a better use of cardiovascular medicines and control of associated Oral adverse effects oral adverse effects. This review sheds lights on the oral adverse effects pertaining to the clinical use of Dry mouth Angioedema cardiovascular drugs. Above and beyond, an adverse correlation between oral disease and cardiovascular Dysgeusia disease has been discussed. -

Pdf (563.04 K)
EGYPTIAN Vol. 65, 927:939, April, 2019 DENTAL JOURNAL I.S.S.N 0070-9484 Orthodontics, Pediatric and Preventive Dentistry www.eda-egypt.org • Codex : 180/1904 THE MOST COMMON 5 PEDIATRIC ORAL LESIONS IN MIDDLE NILE DELTA, EGYPT Talat M. Beltagy*, Enas A. El-Gendy**, Emad F. Essa*** and Ibrahim A. Kabbash**** ABSTRACT Background: The prevalence studies on common pediatric oral lesions (POLs) are still rare compared with those on dental caries and periodontal diseases. POLs vary among different geographic regions, age, racial and lifestyle of each population. The purpose of this study was to determine the most common 5 POLs referred to 5 different dental and medical branches in Middle Nile Delta, Egypt. Materials and methods: A qualitative study design was used depending on expert opinions on oral lesions in children (aged 0-14 years). A total of 1164 dental and medical staff members, dentists and physicians at the hospitals of Universities and Ministry of Health, and Specialized Medical Centers & hospitals in the Middle Nile Delta region were included. The target population of the study was experts in 5 branches: Pedodontics, Oral Medicine and Periodontology, Oral and Maxillofacial Surgery, Pediatrics, and Dermatology and Venereology. Data were collected using a checklist including the common diseases within the scope of the study and each expert was asked to give percentages for children seen with each disease entity in his/her branch. Data analysis: Data were statistically analyzed using Statistical Package for the Social Sciences version 19. For each disease, the number and percentage were calculated and differences between observation recorded by health care workers in University and Ministry of Health were tested by chi-square test. -

Topographical Dermatology Picture Cause Basic Lesion
page: 332 Chapter 12: alphabetical Topographical dermatology picture cause basic lesion search contents print last screen viewed back next Topographical dermatology Alopecia page: 333 12.1 Alopecia alphabetical Alopecia areata Alopecia areata of the scalp is characterized by the appearance of round or oval, smooth, shiny picture patches of alopecia which gradually increase in size. The patches are usually homogeneously glabrous and are bordered by a peripheral scatter of short broken- cause off hairs known as exclamation- mark hairs. basic lesion Basic Lesions: None specific Causes: None specific search contents print last screen viewed back next Topographical dermatology Alopecia page: 334 alphabetical Alopecia areata continued Alopecia areata of the occipital region, known as ophiasis, is more resistant to regrowth. Other hair picture regions can also be affected: eyebrows, eyelashes, beard, and the axillary and pubic regions. In some cases the alopecia can be generalized: this is known as cause alopecia totalis (scalp) and alopecia universalis (whole body). basic lesion Basic Lesions: Causes: None specific search contents print last screen viewed back next Topographical dermatology Alopecia page: 335 alphabetical Pseudopelade Pseudopelade consists of circumscribed alopecia which varies in shape and in size, with picture more or less distinct limits. The skin is atrophic and adheres to the underlying tissue layers. This unusual cicatricial clinical appearance can be symptomatic of cause various other conditions: lupus erythematosus, lichen planus, folliculitis decalvans. Some cases are idiopathic and these are known as pseudopelade. basic lesion Basic Lesions: Atrophy; Scars Causes: None specific search contents print last screen viewed back next Topographical dermatology Alopecia page: 336 alphabetical Trichotillomania Plucking of the hair on a large scale. -
Atrophic Glossitis: Burning Agony of Nutritional Deficiency Anemia 1Neeti Swarup, 2Shreya Gupta, 3Chandrani Sagolsem, 4Zoya Chowdhary, 5Subhash Gupta, 6Nidhi Sinha
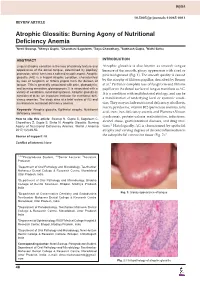
WJOA Neeti Swarup et al 10.5005/jp-journals-10065-0011 REVIEW ARTICLE Atrophic Glossitis: Burning Agony of Nutritional Deficiency Anemia 1Neeti Swarup, 2Shreya Gupta, 3Chandrani Sagolsem, 4Zoya Chowdhary, 5Subhash Gupta, 6Nidhi Sinha ABSTRACT INTRODUCTION Lingual atrophic condition is the loss of ordinary texture and Atrophic glossitis is also known as smooth tongue appearance of the dorsal tongue, determined by papillary because of the smooth, glossy appearance with a red or protrusion, which turns into a soft and smooth aspect. Atrophic pink background (Fig. 1). The smooth quality is caused glossitis (AG) is a lingual atrophic condition, characterized by loss of fungiform or filiform papilla from the dorsum of by the atrophy of filiform papillae, described by Reamy 1 tongue. This is generally associated with pain, glossodynia, et al. Partial or complete loss of fungiform and filiform and burning sensation, glossopyrosis. It is associated with a papillae on the dorsal surface of tongue manifests as AG. variety of conditions, local and systemic. Atrophic glossitis is It is a condition with multifactorial etiology, and can be considered to be an important indicator for nutritional defi- ciency anemias. The study aims at a brief review of AG and a manifestation of underlying local or systemic condi- its relation to nutritional deficiency anemia. tion. They may include nutritional deficiency, riboflavin, niacin, pyridoxine, vitamin B12 (pernicious anemia), folic Keywords: Atrophic glossitis, Epithelial atrophy, Nutritional deficiency anemia. acid, iron (iron deficiency anemia and Plummer-Vinson syndrome), protein-calorie malnutrition, infections, How to cite this article: Swarup N, Gupta S, Sagolsem C, alcohol abuse, gastrointestinal diseases, and drug reac- Chowdhary Z, Gupta S, Sinha N.