Functional Aspects of Floral Nectar Secretion of Ananas Ananassoides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Embriologia De Tillandsia Aeranthos (Lois.) L
UNIVERSIDADE FEDERAL DE SANTA MARIA CENTRO DE CIÊNCIAS NATURAIS E EXATAS PROGRAMA DE PÓS-GRADUAÇÃO EM AGROBIOLOGIA EMBRIOLOGIA DE TILLANDSIA AERANTHOS (LOIS.) L. B. SM. (TILLANDSIOIDEAE- BROMELIACEAE) DISSERTAÇÃO DE MESTRADO Cristiele Spat Santa Maria, RS, Brasil 2012 EMBRIOLOGIA DE TILLANDSIA AERANTHOS (LOIS.) L. B. SM. (TILLANDSIOIDEAE-BROMELIACEAE) Cristiele Spat Dissertação apresentada ao Curso de Mestrado do Programa de Pós-Graduação em Agrobiologia, da Universidade Federal de Santa Maria (UFSM, RS), como requisito parcial para obtenção do grau de Mestre em Agrobiologia Orientador: Prof. Dr. João Marcelo Santos de Oliveira Santa Maria, RS, Brasil 2012 AGRADECIMENTOS À minha família, pelo apoio, incentivo e por compreender as ausências durante esses dois anos. Ao meu Orientador, Prof. Dr. João Marcelo Santos de Oliveira, pela amizade e dedicação durante minha formação, os quais foram fundamentais na execução desse trabalho. Ao Glauber, pelo carinho, apoio e paciência. À Drª. Jaqueline Sarzi Sartori, pela amizade, dedicação, aprendizado e discussões, sempre valiosas, sobre Bromeliaceae Ao César Carvalho de Freitas, pela ajuda e disponibilidade na confecção do material botânico, indispensável na execução deste trabalho. À Marisa Binotto, pela amizade, companherismo e auxílio técnico no laboratório, muito importantes na execução deste estudo. Aos amigos e colegas do Laboratório de Botânica Estrutural, Patrícia, Merielen e Mariane, pelo convívio diário, incentivo e discussões acadêmicas, muito importantes para a realização deste trabalho. Às minhas amigas, Renata, Lara e Letícia, pelos encontros, momentos de descontração e por lembrarem, todos os dias, o valor de uma amizade. À Prof. Drª. Thais Scotti do Canto-Dorow, pela análise taxonômica e disponibilidade em realizar as coletas. -
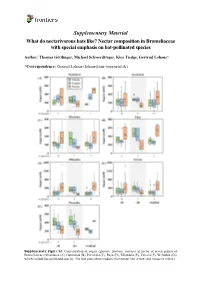
Supplementary Material What Do Nectarivorous Bats Like? Nectar Composition in Bromeliaceae with Special Emphasis on Bat-Pollinated Species
Supplementary Material What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species Author: Thomas Göttlinger, Michael Schwerdtfeger, Kira Tiedge, Gertrud Lohaus* *Correspondence: Gertrud Lohaus ([email protected]) Supplementary Figure S1: Concentration of sugars (glucose, fructose, sucrose) in nectar of seven genera of Bromeliaceae (Alcantarea (A), Guzmania (B), Pitcairnia (C), Puya (D), Tillandsia (E), Vriesea (F), Werauhia (G)) which include bat-pollinated species. The box plots show medians (horizontal line in box) and means (x in box). Supplementary Material What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species Author: Thomas Göttlinger, Michael Schwerdtfeger, Kira Tiedge, Gertrud Lohaus* *Correspondence: Gertrud Lohaus ([email protected]) Supplementary Figure S2: Concentration of amino acids (ala, arg, asn, asp, gaba, gln, glu, gly, his, iso, leu, lys, met, phe, pro, ser, thr, trp, tyr, val) in nectar of seven genera of Bromeliaceae (Alcantarea (A), Guzmania (B), Pitcairnia (C), Puya (D), Tillandsia (E), Vriesea (F), Werauhia (G)), which include bat-pollinated species. The box plots show medians (horizontal line in box) and means (x in box). Supplementary Material What do nectarivorous bats like? Nectar composition in Bromeliaceae with special emphasis on bat-pollinated species Author: Thomas Göttlinger, Michael Schwerdtfeger, Kira Tiedge, Gertrud Lohaus* *Correspondence: Gertrud Lohaus ([email protected]) Supplementary Figure S3: Cation concentrations (Ca2+, K+, Na+, Mg2+) in nectar of seven genera of Bromeliaceae (Alcantarea (A), Guzmania (B), Pitcairnia (C), Puya (D), Tillandsia (E), Vriesea (F), Werauhia (G)), which include bat-pollinated species. The box plots show medians (horizontal line in box) and means (x in box). -

Anatomia Floral De Aechmea Distichantha Lem. E Canistropsis Billbergioides (Schult
Hoehnea 43(2): 183-193, 4 fig., 2016 http://dx.doi.org/10.1590/2236-8906-78/2015 Anatomia floral de Aechmea distichantha Lem. e Canistropsis billbergioides (Schult. & Schult.f) Leme (Bromeliaceae)1 Fernanda Maria Cordeiro de Oliveira2,3, André Melo de Souza2, Brenda Bogatzky Ribeiro Corrêa2, Tatiana Midori Maeda2 e Gladys Flavia Melo-de-Pinna2 Recebido: 13.10.2015; aceito: 26.02.2016 ABSTRACT - (Floral anatomy of Aechmea distichantha Lem. and Canistropsis billbergioides (Schult. & Schult.f) Leme (Bromeliaceae)). Aechmea Ruiz & Pav. and Canistropsis (Mez) Leme belong to the subfamily Bromelioideae, which has the largest morphological diversity in Bromeliaceae. The flower buds of Aechmea distichantha Lem. and Canistropsis billbergioides (Schult. & Schult. f.) Leme were collected, fixed, and processed according to usual techniques in plant anatomy. The species share characteristics such as the presence of spherical crystals of silica in the epidermal cells of perianth; idioblasts with raphids; endothecium with annular thickening; and inferior ovary with axillary placentation. Non- vascular petal appendages were observed only in A. distichantha, arranged in pairs on each petal. Both species present a septal nectary, which nectar is rich in of proteins and carbohydrates. A placental obturator occurs in both species and histochemical tests revealed that the secretion produced by the obturator contains carbohydrates and proteins, probably related to the pollen tube guidance. Keywords: obturator, petal appendages, septal nectary RESUMO - (Anatomia floral de Aechmea distichantha Lem. e Canistropsis billbergioides (Schult. & Schult.f) Leme (Bromeliaceae)). Aechmea Ruiz & Pav. e Canistropsis (Mez) Leme pertencem à subfamília Bromelioideae, detentora da maior diversidade morfológica em Bromeliaceae. Botões florais deAechmea distichantha Lem. -

Plethora of Plants – Collections of the Botanical Garden, Faculty Of
Nat. Croat. Vol. 24(2), 2015 361 NAT. CROAT. VOL. 24 No 2 361–397* ZAGREB December 31, 2015 professional paper / stručni članak – museal collections / muzejske zbirke DOI: 10.302/NC.2015.24.26 PLETHORA OF PLANTS – ColleCtions of the BotaniCal Garden, faCulty of ScienCe, university of ZaGreB (1): temperate Glasshouse exotiCs – HISTORIC OVERVIEW Sanja Kovačić Botanical Garden, department of Biology, faculty of science, university of Zagreb, marulićev trg 9a, HR-10000 Zagreb, Croatia (e-mail: [email protected]) Kovačić, S.: Plethora of plants – collections of the Botanical garden, Faculty of Science, Univer- sity of Zagreb (1): Temperate glasshouse exotics – historic overview. Nat. Croat., Vol. 24, No. 2, 361–397*, 2015, Zagreb due to the forthcoming obligation to thoroughly catalogue and officially register all living and non-living collections in the european union, an inventory revision of the plant collections in Zagreb Botanical Garden of the faculty of science (university of Zagreb, Croatia) has been initiated. the plant lists of the temperate (warm) greenhouse collections since the construction of the first, exhibition Glasshouse (1891), until today (2015) have been studied. synonymy, nomenclature and origin of plant material have been sorted. lists of species grown (or that presumably lived) in the warm greenhouse conditions during the last 120 years have been constructed to show that throughout that period at least 1000 plant taxa from 380 genera and 90 families inhabited the temperate collections of the Garden. today, that collection holds 320 exotic taxa from 146 genera and 56 families. Key words: Zagreb Botanical Garden, warm greenhouse conditions, historic plant collections, tem- perate glasshouse collection Kovačić, S.: Obilje bilja – zbirke Botaničkoga vrta Prirodoslovno-matematičkog fakulteta Sve- učilišta u Zagrebu (1): Uresnice toplog staklenika – povijesni pregled. -

(NOA) : Patrones De Distribución, Prioridades De Conservación Y Cambio Climático Godoy-Bürki, Carolina Doctor En Ciencias Naturales
Naturalis Repositorio Institucional Universidad Nacional de La Plata http://naturalis.fcnym.unlp.edu.ar Facultad de Ciencias Naturales y Museo Diversidad de plantas vasculares en zonas áridas del Noroeste de Argentina (NOA) : patrones de distribución, prioridades de conservación y cambio climático Godoy-Bürki, Carolina Doctor en Ciencias Naturales Dirección: Zuloaga, Fernando O. Co-dirección: Aagesen, Lone Facultad de Ciencias Naturales y Museo 2015 Acceso en: http://naturalis.fcnym.unlp.edu.ar/id/20150319001389 Esta obra está bajo una Licencia Creative Commons Atribución-NoComercial-CompartirIgual 4.0 Internacional Powered by TCPDF (www.tcpdf.org) UNIVERSIDAD NACIONAL DE LA PLATA Facultad de Ciencias Naturales y Museo Diversidad de plantas vasculares en zonas áridas del Noroeste de Argentina (NOA): Patrones de Distribución, Prioridades de Conservación y Cambio climático Tesis presentada para optar al grado de Doctor en Ciencias Naturales de la Universidad Nacional de La Plata Ing. Ana Carolina Godoy-Bürki Director: Dr. Fernando O. Zuloaga Co-directora: Dra. Lone Aagesen 2015 “Todo logro empieza con la decisión de intentarlo.” A mi familia y amigos… Agradecimientos “Cómo empezar sin olvidar a nadie en tan largo camino…” Agradezco con todo el corazón a todos aquellos que me acompañaron en este trayecto de mi vida directa o indirectamente, interesada o desinteresadamente. Gracias por ayudarme a crecer, a florecer, y a madurar para dar, como paso final, el tan anhelado fruto: esta tan querida y por momentos tan odiada tesis doctoral. A mis directores, Dr. Fernando Zuloaga y Dra. Lone Aagesen que me tuvieron gran paciencia en mis momentos difíciles, sin dejar de alentarme ni un solo día. -

Morfo-Anatomia, Ontogenia E Histoquímica De Fruto Em
NATIVIDAD FERREIRA FAGUNDES MORFO-ANATOMIA, ONTOGENIA E HISTOQUÍMICA DE FRUTO EM BROMELIACEAE JUSS. Porto Alegre 2009 i NATIVIDAD FERREIRA FAGUNDES MORFO-ANATOMIA, ONTOGENIA E HISTOQUÍMICA DE FRUTO EM BROMELIACEAE JUSS. Dissertação apresentada ao Programa de Pós- Graduação em Botânica da Universidade Federal do Rio Grande do Sul, como parte dos requisitos para obtenção do Título de Mestre em Botânica. Orientador: Prof. Dr. Jorge Ernesto de Araujo Mariath Porto Alegre 2009 ii Àquela que sempre incentivou e fez tudo para eu pudesse traçar o meu caminho, minha mãe amada, Marlei. iii AGRADECIMENTOS Ao Dr. Jorge Ernesto de Araujo Mariath, pelas sugestões e críticas fundamentais, pela compreensão, pela confiança e por se mostrar sempre tão atencioso e disposto, mesmo com tantos compromissos. Aos queridos colegas do Laboratório de Anatomia Vegetal Adriano Silvério, Aline Tonin, Carla de Pelegrin, Daniele Rodrigues, Denise Klein, Érica Duarte, Fernanda Silva, Greta Dettke e à técnica Juliana Troleis, pela amizade, companheirismo e solicitude e pelos valiosos aprendizados, teóricos ou práticos. À Dra. Alexandra Antunes Mastroberti e ao Dr. Rinaldo Pires dos Santos, pela disponibilidade em ajudar sempre. Aos professores e alunos do Programa de Pós-Graduação em Botânica, pelos ensinamentos, reflexões e trocas de informações. À Fundação Zoobotânica do Rio Grande do Sul, pela autorização de coleta na Coleção de Bromeliaceae do Jardim Botânico de Porto Alegre; e, mais especificamente, à Dra. Andréia Carneiro, curadora, e aos funcionários da Coleção, pela atenção durante as coletas. Ao Prof. Dr. Luís Rios de Moura Baptista, pela companhia e receptividade nas saídas a Dom Pedro de Alcântara, em terrenos de sua propriedade. -

The Floral Anatomy of Yacca Gloriosal. (Agavaceae) with a Note on Taxonomic Position of Tiie Geiyus
J. Plytol. Res. l9Q):209-214,2N6 THE FLORAL ANATOMY OF YACCA GLORIOSAL. (AGAVACEAE) WITH A NOTE ON TAXONOMIC POSITION OF TIIE GEIYUS D-1,. PATIL and RM.PAIT P.G.Department of Botany, S.S.V.PS's L.K.Dr.PR.Ghogrry sciencc college, Dhule-424005, tndia. '24, Swanandnagar, Ncar Chetananagar, Aurangabad-43|oo5, lndia. The floral anato my gf Yu:ca gtoriosa L. is presented. The outer florat whorls are shortly adnate to the base ofovary. This is inferred as a trend towards the developmurt ofan inferior ovary. The fiicarpellary syncarpous g/noecium is unilocular in the basal part and trilocular upwards. The placenAtion in the basal part though appears parietal, it is transitional leading to the axile type in the upper part ofthe ovary. The carpets are basically 5-traced. The association of the placental bundles with the septal nectaries is reiterated. The extension ofthe rcsidual placental bundles in the styte is phylogenetically insignificant. The outer and inner perianth segments are S-traced and 3-traced respeciively. The stamens are l-traced organs. Placement ofthe genus Yucca under the Agavaceae isjustified on various grounds. Keywords : Floral anatomy; Taxonomy; Yucca glorioso. Introduction MS, OS and D strands, another divides into the MS and positio-n Systematic of the genus Yucca has been much OS-D bundles with the latter splitting quickly inro the debated in the various contemporary systems of plantr{2. constituent strands (Fig.4), MP-IS bundles separate out lnthe earlidir communicalions, the floral anatomyofsome intorthrreomponedt strands a little upwards (Fig.5). The agavoids was describedrr-r8. -

The Floral Anatomy of <Emphasis Type="Italic">Puya Spathacea
Proc. Indiaa Aear Sed. (Plartt Sed.), Vol. 91, Number 6, December 1982, pp. 473-478. I~) Printed in India. The floral anatomy of Puya spathacea Mez. (Bromeliaceae) with special reference to nectaries R A KULKARNI and R M PA[ Plant Morphol6gy Laboratory, Department of Botany, Marathwada University, Autrsngabad 431 004, India MS reeeived 25 May 1981 Abstraet. The floral anatomy of Puya spathacea /V[ez. is dr in detail. The outer floral whorls ate united, to develop a sltort hypanthium whieh is actnate to the baso of the ovary. The sopals aro five-traced, anct the petals, three-traeed. The placentation is axile. The oecurrenee of numerous avules in more than two rows as well as the extension of the earpellary ventrals into the style ate less advanced features. The avarian neetary is extensively developed and shows a transition between typic,3l septal and epigynous neetaries of aertain monoeoty- ledonous taxa. Keywords. Puya spathacea ; floral anatomy ; nectaries. 1. Introduction The Bromeliaoeae ate a fairly largo family with abaut sixty g~aera and about 2000 speeies. Hutchinson (1959) eonsiders the family to be a homogeneous taxon reprosenting the ' clŸ of a lino of deseent wherein the oalyx and oorolla have remained distinet of fairly distinct from eaoh other '. He treats ir as related to, but more advanr than the Commelinales. Smith's extensive studies (1934) point out that the family has strongest affinities with the Rapateaeeae and that both families probably arose from a eommon ancestral stoek. Within the family, Puya is treated as probably the souroe of ancestral types from whioh the other sub-famŸ developed. -

CACTUS COURIER Newsletter of the Palomar Cactus and Succulent Society the North San Diego County Cactus and Succulent Society
CACTUS COURIER Newsletter of the Palomar Cactus and Succulent Society The North San Diego County Cactus and Succulent Society Volume 62, Number 3 March 2016 NEXT MEETING th Spring Member Festival Saturday, March 26 Park Ave. Community Center Saturday, March 26, 2015 11am to 3:00pm 210 Park Ave., Escondido • We are once again having our Spring Member Festival to Brag Plants, Exchange Table, Benefit Drawing - as usual! showcase the many members who are just starting out, and those who have been doing it for a while as a relaxing 11:00am – 3:00pm hobby. • The Festival will take place in our usual meeting room. You will be able to wander freely between activities. MEMBERSHIP • The judging and workshops will run consecutively so you RENEWAL don’t have to miss a thing. If you have not yet renewed WE WILL HAVE THE FOLLOWING: your membership, it’s time! - Member Plant Sale To continue as a member and - Plant Show to receive the next newsletter you - Workshops MUST RENEW YOUR MEMBERSHIP! - Refreshments Please do so at the March meeting, or mail it in to Leon at the address on the Membership Form by 3/31. See page 11 for form and address. IN THIS ISSUE Spring Member Festival p. 1-3 Refreshments p. 3 Brag Plant Winners p. 4 News of the Safari Park Gardens p. 5 - Volunteers Lifetime Member Award p. 6 Club T-shirt Contest p. 6 Save the Date p. 7 New Feature – Brag Photos p. 8 Garden Tour, Conference flyers p. 9-10 Club Misc. Info p. -

Ovary Structure in the Costaceae (Zingiberales)
Ovary Structure in the Costaceae (Zingiberales) S. Winnell H. Newman, Bruce K. Kirchoff International Journal of Plant Sciences, Volume 153, Issue 3, Part 1 (Sep., 1992), 471-487. Newman, S. W. and B. K. Kirchoff. 1992. Ovary structure in the Costaceae (Zingiberales). International Journal of the Plant Sciences 153: 471-487. Made available courtesy of University of Chicago Press: http://www.journals.uchicago.edu/doi/abs/10.1086/297054 Your use of the JSTOR archive indicates your acceptance of JSTOR' s Terms and Conditions of Use, available at http://www.jstor.org/about/terms.html. JSTOR' s Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. International Journal of Plant Sciences is published by University of Chicago Press. Please contact the publisher for further permissions regarding the use of this work. Publisher contact information may be obtained at http://www.jstor.org/journals/ucpress.html. International Journal of Plant Sciences ©1992 University of Chicago Press JSTOR and the JSTOR logo are trademarks of JSTOR, and are Registered in the U.S. Patent and Trademark Office. For more information on JSTOR contact [email protected]. ©2001 JSTOR http://www.jstor.org/ Tue Oct 9 12:21:17 2001 Int. -

Fernanda Maria Cordeiro De Oliveira Contribuições Aos Estudos Anatômicos De Bromeliaceae
Fernanda Maria Cordeiro de Oliveira Contribuições aos estudos anatômicos de Bromeliaceae (Poales) sob uma perspectiva filogenética Contribution to the anatomical studies of Bromeliaceae (Poales) under a phylogenetic perspective São Paulo 2017 Fernanda Maria Cordeiro de Oliveira Contribuições aos estudos anatômicos de Bromeliaceae (Poales) sob uma perspectiva filogenética Contribution to the anatomical studies of Bromeliaceae (Poales) under a phylogenetic perspective Tese apresentada ao Instituto de Biociências da Universidade de São Paulo, para a obtenção de Título de Doutora em Ciências Biológicas, na Área de Botânica. Orientadora: Profa. Dra. Gladys Flavia de Albuquerque Melo de Pinna Co-Orientadora: Profa. Dra. Maria das Graças Wanderley. São Paulo 2017 Oliveira, Fernanda Maria Cordeiro Contribuições aos estudos anatômicos de Bromeliaceae (Poales) sob uma perspectiva filogenética 161 páginas Tese (Doutorado) - Instituto de Biociências da Universidade de São Paulo. Departamento de Botânica. 1. Reconstrução de caracteres ancestrais; 2. Evolução; 3. Complexo Nidularióide; 4. Anatomia floral; 5. Tricomas glandulares I Universidade de São Paulo. Instituto de Biociências. Departamento de Botânica. Comissão Julgadora _______________________________ _______________________________ Prof(a). Dr(a). Prof(a). Dr(a). _______________________________ _______________________________ Prof(a). Dr(a). Prof(a). Dr(a). _______________________________ Profa. Dra. Gladys Flavia de Albuquerque Melo de Pinna (Orientadora) Àquela que me amou desde o primeiro olhar. “A humanidade é parte de um vasto universo em evolução. A Terra, nosso lar, está viva com uma comunidade de vida única. As forças da natureza fazem da existência uma aventura exigente e incerta, mas a Terra providenciou as condições essenciais para a evolução da vida. (...) O meio ambiente global com seus recursos finitos é uma preocupação comum de todas as pessoas. -

Network Scan Data
Selbyana 20(2): 201-223. 1999. CHECKLIST OF BOLIVIAN BROMELIACEAE WITH NOTES ON SPECIES DISTRIBUTION AND LEVELS OF ENDEMISM THORSTEN KROMER AND MICHAEL KESSLER I Albrecht-von-Haller Institut ffir Pflanzenwissenschaften der Universitat Gottingen, Abteilung Systematische Botanik, Untere Karsptile 2, Gottingen, D-37073. E-mail forMK:[email protected] BRUCE K. HOLST* AND HARRY E. LUTHER The Marie Selby Botanical Gardens, 811 South Palm Ave., Sarasota, FL 34236 USA. E-mail for BKH: [email protected] ERIC J. GOUDA University Botanic Gardens, P.O. Box 80.162, NL-3508 TD Utrecht, The Netherlands E-mail: [email protected] PIERRE L. I1nsCH Fundaci6n Amigos de la NaturalezaIBotanical Institute of the University of Bonn, Germany, Casilla 2241, Santa Cruz, Bolivia. E-mail: [email protected] WALTER TILL Botanical Institute of the University of Vienna, Rennweg 14, A-1030, Wien, Austria E-mail: [email protected] ROBERTO V A.SQUEZ Sociedad Boliviana de Botanica, Casilla 3822, Santa Cruz, Bolivia E-mail: [email protected] ABSTRACT. A discussion of Bromeliaceae diversity in Bolivia, and a checklist of the 21 genera and 281 species occuring there is presented. Each species entry in the checklist includes accepted name, compre hensive synonymy for all species described based on Bolivian types, additional pertinent synonymy, ele vation range above sea level, distribution by department, and an indication of which species are endemic to Bolivia. RESUMEN. Aqui se describe la diversidad de la familia Bromeliaceae en Bolivia. Se incluye un listado de los 21 generos y 281 especies que occurren ahi.