Renato Christensen Nali
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Multilocus Analysis of the Catfish Family Trichomycteridae (Teleostei: Ostario- Physi: Siluriformes) Supporting a Monophyletic Trichomycterinae
Accepted Manuscript Multilocus analysis of the catfish family Trichomycteridae (Teleostei: Ostario- physi: Siluriformes) supporting a monophyletic Trichomycterinae Luz E. Ochoa, Fabio F. Roxo, Carlos DoNascimiento, Mark H. Sabaj, Aléssio Datovo, Michael Alfaro, Claudio Oliveira PII: S1055-7903(17)30306-8 DOI: http://dx.doi.org/10.1016/j.ympev.2017.07.007 Reference: YMPEV 5870 To appear in: Molecular Phylogenetics and Evolution Received Date: 28 April 2017 Revised Date: 4 July 2017 Accepted Date: 7 July 2017 Please cite this article as: Ochoa, L.E., Roxo, F.F., DoNascimiento, C., Sabaj, M.H., Datovo, A., Alfaro, M., Oliveira, C., Multilocus analysis of the catfish family Trichomycteridae (Teleostei: Ostariophysi: Siluriformes) supporting a monophyletic Trichomycterinae, Molecular Phylogenetics and Evolution (2017), doi: http://dx.doi.org/10.1016/ j.ympev.2017.07.007 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Multilocus analysis of the catfish family Trichomycteridae (Teleostei: Ostariophysi: Siluriformes) supporting a monophyletic Trichomycterinae Luz E. Ochoaa, Fabio F. Roxoa, Carlos DoNascimientob, Mark H. Sabajc, Aléssio -

Water Diversion in Brazil Threatens Biodiversit
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/332470352 Water diversion in Brazil threatens biodiversity Article in AMBIO A Journal of the Human Environment · April 2019 DOI: 10.1007/s13280-019-01189-8 CITATIONS READS 0 992 12 authors, including: Vanessa Daga Valter Monteiro de Azevedo-Santos Universidade Federal do Paraná 34 PUBLICATIONS 374 CITATIONS 17 PUBLICATIONS 248 CITATIONS SEE PROFILE SEE PROFILE Fernando Pelicice Philip Fearnside Universidade Federal de Tocantins Instituto Nacional de Pesquisas da Amazônia 68 PUBLICATIONS 2,890 CITATIONS 612 PUBLICATIONS 20,906 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Freshwater microscrustaceans from continental Ecuador and Galápagos Islands: Integrative taxonomy and ecology View project Conservation policy View project All content following this page was uploaded by Philip Fearnside on 11 May 2019. The user has requested enhancement of the downloaded file. The text that follows is a PREPRINT. O texto que segue é um PREPRINT. Please cite as: Favor citar como: Daga, Vanessa S.; Valter M. Azevedo- Santos, Fernando M. Pelicice, Philip M. Fearnside, Gilmar Perbiche-Neves, Lucas R. P. Paschoal, Daniel C. Cavallari, José Erickson, Ana M. C. Ruocco, Igor Oliveira, André A. Padial & Jean R. S. Vitule. 2019. Water diversion in Brazil threatens biodiversity: Potential problems and alternatives. Ambio https://doi.org/10.1007/s13280-019- 01189-8 . (online version published 27 April 2019) ISSN: 0044-7447 (print version) ISSN: 1654-7209 (electronic version) Copyright: Royal Swedish Academy of Sciences & Springer Science+Business Media B.V. -

Universidade De Brasília Instituto De Ciências Biológicas Programa De
Universidade de Brasília Instituto de Ciências Biológicas Programa de Pós-graduação em Ecologia DISTRIBUIÇÃO E CONSERVAÇÃO DE ANFÍBIOS NO CERRADO EM CENÁRIOS ATUAIS E FUTUROS Débora Leite Silvano Brasília - DF Abril de 2011 Universidade de Brasília Instituto de Ciências Biológicas Programa de Pós-graduação em Ecologia DISTRIBUIÇÃO E CONSERVAÇÃO DE ANFÍBIOS NO CERRADO EM CENÁRIOS ATUAIS E FUTUROS Débora Leite Silvano Tese apresentada ao Programa de Pós-Graduação em Ecologia da Universidade de Brasília como parte das exigências para obtenção do título de Doutor em Ecologia. Orientador: Prof. Dr. Guarino Rinaldi Colli Brasília - DF Abril de 2011 À razão da minha mais completa e autêntica felicidade Rinaldo e Melissa AGRADECIMENTOS Muitas pessoas e instituições contribuíram de maneira direta ou indireta para este trabalho e eu não conseguiria citar individualmente cada uma, mas agradeço imensamente a todas elas. Agradeço especialmente: Ao meu marido e grande companheiro Rinaldo Pereira por ter sempre me apoiado incondicionalmente, sendo pai e mãe em todas as minhas longas e frequentes ausências nestes últimos quatro anos, e à minha filha Melissa por ser tão madura, no alto dos seus cinco aninhos, suportando tão bem a minha ausência; À minha parceira neste trabalho, e agora grande amiga, Paula Valdujo , por todas as infinitas horas de trabalho que compartilhamos, por todas as ideias trocadas e por todo apoio a qualquer hora e momento. Sem essa parceria nada disso seria possível; Ao meu orientador Guarino Colli , por ter me deixado caminhar -
For Review Only
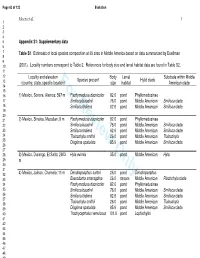
Page 63 of 123 Evolution Moen et al. 1 1 2 3 4 5 Appendix S1: Supplementary data 6 7 Table S1 . Estimates of local species composition at 39 sites in Middle America based on data summarized by Duellman 8 9 10 (2001). Locality numbers correspond to Table 2. References for body size and larval habitat data are found in Table S2. 11 12 Locality and elevation Body Larval Subclade within Middle Species present Hylid clade 13 (country, state, specific location)For Reviewsize Only habitat American clade 14 15 16 1) Mexico, Sonora, Alamos; 597 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 17 Smilisca baudinii 76.0 pond Middle American Smilisca clade 18 Smilisca fodiens 62.6 pond Middle American Smilisca clade 19 20 21 2) Mexico, Sinaloa, Mazatlan; 9 m Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 22 Smilisca baudinii 76.0 pond Middle American Smilisca clade 23 Smilisca fodiens 62.6 pond Middle American Smilisca clade 24 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 25 Diaglena spatulata 85.9 pond Middle American Smilisca clade 26 27 28 3) Mexico, Durango, El Salto; 2603 Hyla eximia 35.0 pond Middle American Hyla 29 m 30 31 32 4) Mexico, Jalisco, Chamela; 11 m Dendropsophus sartori 26.0 pond Dendropsophus 33 Exerodonta smaragdina 26.0 stream Middle American Plectrohyla clade 34 Pachymedusa dacnicolor 82.6 pond Phyllomedusinae 35 Smilisca baudinii 76.0 pond Middle American Smilisca clade 36 Smilisca fodiens 62.6 pond Middle American Smilisca clade 37 38 Tlalocohyla smithii 26.0 pond Middle American Tlalocohyla 39 Diaglena spatulata 85.9 pond Middle American Smilisca clade 40 Trachycephalus venulosus 101.0 pond Lophiohylini 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 Evolution Page 64 of 123 Moen et al. -

The Amphibians of São Paulo State, Brazil Amphibians of São Paulo Biota Neotropica, Vol
Biota Neotropica ISSN: 1676-0611 [email protected] Instituto Virtual da Biodiversidade Brasil Santos Araújo, Olívia Gabriela dos; Toledo, Luís Felipe; Anchietta Garcia, Paulo Christiano; Baptista Haddad, Célio Fernando The amphibians of São Paulo State, Brazil amphibians of São Paulo Biota Neotropica, vol. 9, núm. 4, 2009, pp. 197-209 Instituto Virtual da Biodiversidade Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=199114284020 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Biota Neotrop., vol. 9, no. 4 The amphibians of São Paulo State, Brazil amphibians of São Paulo Olívia Gabriela dos Santos Araújo1,4, Luís Felipe Toledo2, Paulo Christiano Anchietta Garcia3 & Célio Fernando Baptista Haddad1 1Departamento de Zoologia, Instituto de Biociências, Universidade Estadual Paulista – UNESP, CP 199, CEP 13506-970, Rio Claro, SP, Brazil 2Museu de Zoologia “Prof. Adão José Cardoso”, Universidade Estadual de Campinas – UNICAMP, Rua Albert Einstein, s/n, CEP 13083-863, Campinas, SP, Brazil, e-mail: [email protected] 3Departamento de Zoologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais – UFMG, Av. Antônio Carlos, 6627, Pampulha, CEP 31270-901, Belo Horizonte, MG, Brazil 4Corresponding author: Olívia Gabriela dos Santos Araújo, e-mail: [email protected] ARAÚJO, O.G.S., TOLEDO, L.F., GARCIA, P.C.A. & HADDAD, C.F.B. The amphibians of São Paulo State. Biota Neotrop. 9(4): http://www.biotaneotropica.org.br/v9n4/en/abstract?inventory+bn03109042009. -

Sobre a BIOTA NEOTROPICA
Biota Neotrop. vol. 9, no. 1, jan./mar. 2009 ISSN 1678-6424 ISSN 1678-6424 vol. 9, no. 1, jan./mar. 2009 jan./mar. 1, no. 9, vol. Sumário Artigos Uma revisão da distribuição de Ocotea curucutuensis J.B. Baitello na região sudeste do Brasil vol. 9, no. 1, jan./mar. 2009 Frederico Alexandre Roccia Dal Pozzo Arzolla, João Batista Baitello, George John Shepherd, Gláucia Cortez Ramos de Paula & Ricardo Bertoncello ...............................................21 Novos registros, sinonímia e descrição do macho de Culicoides horticola Lutz (Diptera: Ceratopogonidae) Maria Luiza Felippe-Bauer & Gustavo Ricardo Spinelli .....................................................................................................................................................................................................27 Os pequenos mamíferos da altamente impactada Floresta Atlântica do Nordeste do Brasil, Centro de Endemismo Pernambuco Paulo Henrique Asfora & Antonio Rossano Mendes Pontes ..............................................................................................................................................................................................31 Ácaros associados ao cafeeiro (Coffea spp.) no estado de São Paulo, Brasil. Parte I. Mesostigmata Jeferson Luiz de Carvalho Mineiro, Adalton Raga, Mario Eidi Sato & Antonio Carlos Lofego ...........................................................................................................................................37 Relações entre diversidade íctia e fatores hidrodinâmicos -

A New Species of Trichogenesfrom the Rio Itapemirim Drainage
Neotropical Ichthyology, 8(4):707-717, 2010 Copyright © 2010 Sociedade Brasileira de Ictiologia A new species of Trichogenes from the rio Itapemirim drainage, southeastern Brazil, with comments on the monophyly of the genus (Siluriformes: Trichomycteridae) Mário C. C. de Pinna1, José Luiz Helmer2, Heraldo A. Britski1 and Leandro Rodrigues Nunes2 A new species of the formerly monotypic genus Trichogenes is described from a high-altitude stream of the rio Itapemirim system, an isolated Atlantic drainage in the State of Espírito Santo, southeastern Brazil. Trichogenes claviger, new species, differs from all other trichomycterids by the sexually dimorphic posterior process of the opercle, much elongated in males; the terminal mouth; the deeply bifurcated anterior neural spines and the presence of a large anterodorsal claw-like process on the neural arches of the anterior four free vertebrae. The new species also differs from its only congener, T. longipinnis, by a number of additional traits, including the the lack of branched anal-fin rays in specimens of any size; the broader than long posterior nostril; the deeper head (head depth 72.9-86.6% HL); the presence of a fine dark line along the base of the anal fin; the lack of dark spots on cheeks; the shape of the interopercle; the presence of odontodes on a bony expansion on the posterodorsal margin of the interopercle; the fewer vertebrae (35); the absence of an antorbital; and the fewer pleural ribs (eight). Small juveniles of the new species are also strikingly different from those of all other Trichomycteridae, including T. longipinnis, having a very large lateral eye, an upturned mouth, and compressed head. -

Thesis-1968-A753m.Pdf (1.124Mb)
THE MELOIDAE (COLEOPTERA) OF OKLAHOMA By DONALD CHESTER ARNOLD •I Bachelor of Science Oklahoma State University Stillwater, Oklahoma 1964 Submitted to the faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE July, 1968 ll I ) OKLAHOMA STATE UNIVERSITY LIBRARY JAN i81969 THE MELOIDAE (COLEOPTERA) OF OKLAHOMA Thesis Approved: Dean of the Graduate College ii Pl:lEFACE This thesis, a local fauna study1 contains keys, descriptions, dis tribution records, and larval and adult p.ost records for the blister beetles of Oklahoma. A total of sixty-six species and subspecies is known to occur in the state. Four others are included because their distribution is such that they should occur here. I wish to thank my rnajor adviser, Pr. W. A. Drew, and the other members of my advisory committee, D:rs. D. E~ Howell, J. H. Young, and U. T. Waterfall for criticism of the manuscript. I would also like to thank Dr. C. E. Hopla, Stovall Museum, University of Oklahoma, for the use of facilities and specimens; Dr. F .. G. Werner, University of Arizona, Dr. lL B. Selander, University of Illinois, Dr. W. R. Enns, University of Missouri, Dr. J. M. Mathieu, Instituto Tecnol ogico de Monterrey, an<;l Mr. J. D. :Pinto, Un~versity of Illinois, for help in determining specimensi and the following for ~ollE;lcting speci- . mens: J. H. Young, D. G. BottreU, K. F. Schaefer, and R. D. Eikenbary. Special thanks are expressed to my wife, Judy, for typing and for her patience, under standing, and encouragement during the course of this study and Mrs. -

A Importância De Se Levar Em Conta a Lacuna Linneana No Planejamento De Conservação Dos Anfíbios No Brasil
UNIVERSIDADE FEDERAL DE GOIÁS INSTITUTO DE CIÊNCIAS BIOLÓGICAS PROGRAMA DE PÓS-GRADUAÇÃO EM ECOLOGIA E EVOLUÇÃO A IMPORTÂNCIA DE SE LEVAR EM CONTA A LACUNA LINNEANA NO PLANEJAMENTO DE CONSERVAÇÃO DOS ANFÍBIOS NO BRASIL MATEUS ATADEU MOREIRA Goiânia, Abril - 2015. TERMO DE CIÊNCIA E DE AUTORIZAÇÃO PARA DISPONIBILIZAR AS TESES E DISSERTAÇÕES ELETRÔNICAS (TEDE) NA BIBLIOTECA DIGITAL DA UFG Na qualidade de titular dos direitos de autor, autorizo a Universidade Federal de Goiás (UFG) a disponibilizar, gratuitamente, por meio da Biblioteca Digital de Teses e Dissertações (BDTD/UFG), sem ressarcimento dos direitos autorais, de acordo com a Lei nº 9610/98, o do- cumento conforme permissões assinaladas abaixo, para fins de leitura, impressão e/ou down- load, a título de divulgação da produção científica brasileira, a partir desta data. 1. Identificação do material bibliográfico: [x] Dissertação [ ] Tese 2. Identificação da Tese ou Dissertação Autor (a): Mateus Atadeu Moreira E-mail: ma- teus.atadeu@gm ail.com Seu e-mail pode ser disponibilizado na página? [x]Sim [ ] Não Vínculo empregatício do autor Bolsista Agência de fomento: CAPES Sigla: CAPES País: BRASIL UF: D CNPJ: 00889834/0001-08 F Título: A importância de se levar em conta a Lacuna Linneana no planejamento de conservação dos Anfíbios no Brasil Palavras-chave: Lacuna Linneana, Biodiversidade, Conservação, Anfíbios do Brasil, Priorização espacial Título em outra língua: The importance of taking into account the Linnean shortfall on Amphibian Conservation Planning Palavras-chave em outra língua: Linnean shortfall, Biodiversity, Conservation, Brazili- an Amphibians, Spatial Prioritization Área de concentração: Biologia da Conservação Data defesa: (dd/mm/aaaa) 28/04/2015 Programa de Pós-Graduação: Ecologia e Evolução Orientador (a): Daniel de Brito Cândido da Silva E-mail: [email protected] Co-orientador E-mail: *Necessita do CPF quando não constar no SisPG 3. -

Download PDF (Inglês)
Biota Neotropica 18(3): e20170322, 2018 www.scielo.br/bn ISSN 1676-0611 (online edition) Article Anuran amphibians in state of Paraná, southern Brazil Manuela Santos-Pereira1* , José P. Pombal Jr.2 & Carlos Frederico D. Rocha1 1Universidade do Estado do Rio de Janeiro, Ecologia, Rua São Francisco Xavier, 524, Rio de Janeiro, RJ, Brasil 2Universidade Federal do Rio de Janeiro, Museu Nacional, Departamento de Vertebrados, Rio de Janeiro, RJ, Brasil *Corresponding author: Manuela Santos-Pereira, e-mail: [email protected] SANTOS-PEREIRA, M., POMBAL Jr., J.P., ROCHA, C.F.D. Anuran amphibians in state of Paraná, southern Brazil. Biota Neotropica. 18(3): e20170322. http://dx.doi.org/10.1590/1676-0611-BN-2017-0322 Abstract: The state of Paraná, located in southern Brazil, was originally covered almost entirely by the Atlantic Forest biome, with some areas of Cerrado savanna. In the present day, little of this natural vegetation remains, mostly remnants of Atlantic Forest located in the coastal zone. While some data are available on the anurans of the state of Paraná, no complete list has yet been published, which may hamper the understanding of its potential anuran diversity and limit the development of adequate conservation measures. To rectify this situation, we elaborated a list of the anuran species that occur in state of Paraná, based on records obtained from published sources. We recorded a total of 137 anuran species, distributed in 13 families. Nineteen of these species are endemic to the state of Paraná and five are included in the red lists of the state of Paraná, Brazil and/or the IUCN. -

The Meloidae
UC Riverside UC Riverside Previously Published Works Title The Meloidae (Coleoptera) of Australasia: A generic review, descriptions of new taxa, and a challenge to the current definition of subfamilies posed by exceptional variation in male genitalia Permalink https://escholarship.org/uc/item/1zv246c2 Journal Invertebrate Systematics, 27(4) ISSN 1445-5226 Authors Bologna, MA Turco, F Pinto, JD Publication Date 2013-09-09 DOI 10.1071/IS12054 Peer reviewed eScholarship.org Powered by the California Digital Library University of California CSIRO PUBLISHING Invertebrate Systematics, 2013, 27, 391–427 http://dx.doi.org/10.1071/IS12054 The Meloidae (Coleoptera) of Australasia: a generic review, descriptions of new taxa, and a challenge to the current definition of subfamilies posed by exceptional variation in male genitalia M. A. BolognaA,D, F. Turco B and J. D. Pinto C ADipartimento di Scienze, Università Roma Tre, Viale G. Marconi 446, 00146 Roma, Italy. BQueensland Museum, Natural Environments Program, Entomology, PO Box 3300, South Brisbane, Qld 4101, Australia. CDepartment of Entomology, University of California, Riverside, CA 92521, USA. DCorresponding author. Email: [email protected] Abstract. The seven Australasian genera of blister beetles (Coleoptera : Meloidae : Nemognathinae) are reviewed. Included are a key to genera, generic synopses and descriptions of two new genera of Nemognathini, Australozonitis and Pulchrazonitis, as well as a new monotypic tribe Palaestrini, which features a bauplan of male genitalia unique not only to the subfamily Nemognathinae but to the entire family. The genus Palaestra is redefined to include several Australasian, Asian and African species previously assigned to Zonitis. Exceptional variation of male genitalia encountered in the Palaestrini challenges current subfamily definitions, which are partly based on male genitalic structure and correlated sexual behaviour. -

Instituto Amigos Da Reserva Da Biosfera Da Mata Atlântica
INSTITUTO AMIGOS DA RESERVA DA BIOSFERA DA MATA ATLÂNTICA Realização Execução Apoio INSTITUTO AMIGOS DA RESERVA DA BIOSFERA DA MATA ATLÂNTICA PROJETO TCCA/FF MOSAICO PARANAPIACABA 5.1.2. Estudo técnico especializado com indicação de proposta para ampliação, adequação ou criação de áreas naturais protegidas Gleba Lageado - Jeremias Produto II – Relatório Consolidado e Proposta IA-RBMA Março 2014 Realização Execução Apoio INSTITUTO AMIGOS DA RESERVA DA BIOSFERA DA MATA ATLÂNTICA Realização: Fundação para a Conservação e a Produção Florestal do Estado de São Paulo – Fundação Florestal Secretaria de Meio Ambiente do Estado de São Paulo - SMA Execução: Instituto Amigos da Reserva da Biosfera da Mata Atlântica – IA-RBMA Coordenação Geral: Fundação Florestal Jeannette Vieira Geenen – coordenação Kátia Regina Pisciotta Ivaldo José Santos Braz Maria Aparecida Resende Gestores das UC´s do Mosaico de Paranapiacaba: PEI, PETAR, PECB, PENAP, EE Xituê e APA dos Quilombos do Médio Ribeira IA - RBMA Clayton Ferreira Lino – Presidente e Coordenação Geral Nelson Antônio Calil Filho – Coordenação Técnica Nilson Máximo de Oliveira – Coordenação Executiva Realização Execução Apoio INSTITUTO AMIGOS DA RESERVA DA BIOSFERA DA MATA ATLÂNTICA Consultorias Técnicas Especializadas envolvidas nos Projetos: PROJETO MOSAICO PARANAPIACABA – TCCA/FF: Coordenação Geral: Clayton Ferreira Lino – Presidente IA-RBMA Coordenação Técnica: Nelson Antonio Calil Filho Coordenação Temática: Kátia Carolino – Sistema Fundiário José Antonio Basso Scaleante – Uso Público Kátia Mazzei - Geoprocessamento Marcos Melo – Ocupação Antrópica Nelson Antonio Calil Filho – Meio Biótico Nilson Máximo de Oliveira – Mosaicos Sérgio Serafini Júnior – Meio Físico Carlos Eduardo Martins - Meio Físico - Aspectos do Carste Equipe executora: Meio Biótico - Meio Ambiente Consult Msc. Nelson Antônio Calil Filho – coordenador Dr.