Innovation Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Are Nanodiamond-Encrusted Teeth the Future of Dental Implants? 18 September 2013, by Brianna Deane
Are nanodiamond-encrusted teeth the future of dental implants? 18 September 2013, by Brianna Deane prosthetic joints or teeth, which leads to the implants becoming loose—or failing. Implant failures necessitate additional procedures, which can be painful and expensive, and can jeopardize the function the patient had gained with an implant. These challenges are exacerbated when the disease occurs in the mouth, where there is a limited supply of local bone that can be used to secure the prosthetic tooth, a key consideration for both functional and aesthetic reasons. The study, led by Dr. Dean Ho, professor of oral biology and medicine and co-director of the Jane and Jerry Weintraub Center for Reconstructive Biotechnology at the UCLA School of Dentistry, Nanodiamonds. Credit: UCLA School of Dentistry appears online in the peer-reviewed Journal of Dental Research. During bone repair operations, which are typically (Medical Xpress)—UCLA researchers have costly and time-consuming, doctors insert a sponge discovered that diamonds on a much, much through invasive surgery to locally administer smaller scale than those used in jewelry could be proteins that promote bone growth, such as bone used to promote bone growth and the durability of morphogenic protein. dental implants. Ho's team discovered that using nanodiamonds to Nanodiamonds, which are created as byproducts deliver these proteins has the potential to be more of conventional mining and refining operations, are effective than the conventional approaches. The approximately four to five nanometers in diameter study found that nanodiamonds, which are invisible and are shaped like tiny soccer balls. to the human eye, bind rapidly to both bone morphogenetic protein and fibroblast growth factor, Scientists from the UCLA School of Dentistry, the demonstrating that the proteins can be UCLA Department of Bioengineering and simultaneously delivered using one vehicle. -

2019-2020 UCLA Academic Senate Program Review of University Extension
2019-2020 ACADEMIC SENATE PROGRAM REVIEW OF UNIVERSITY EXTENSION Internal Reviewers Olga T. Yokoyama, Humanities, Graduate Council, Review Team Chair Simon Board, Economics, Undergraduate Council External Reviewers Susan Greenbaum, Dean, Professional Studies, New York University John LaBrie, Dean, Professional Studies, Clark University Date of Site Visit: January 14-15, 2020 Approved by Undergraduate Council: April 10, 2020 Approved by Graduate Council: May 1, 2020 Appendix I: External Reviewers’ Report Appendix II: Site Visit Schedule Please note: The Self-Review report was previously distributed. If you need a copy, please contact the Academic Senate Office at [email protected]. 2019-2020 ACADEMIC SENATE PROGRAM REVIEW OF UNIVERSITY EXTENSION Introduction This report of the Internal Review Team for the 2019-2020 review of University Extension (UNEX) follows eight years after the prior Academic Senate Program Review of UNEX in 2011-2012. The current review draws primarily on a two-day site visit held on January 14-15, 2020, the prior Academic Senate Program Review Report from 2011-2012, the UNEX self-review of 2019, and the Huron Report of 2018; for the figures and other specific information, we relied on the Self- Review report and other data provided by the Academic Senate. We concur with the previous two reports in that “this is a somewhat daunting task, because of the sheer size of the UNEX enterprise”. This review comes at a time of transition. The new Dean Eric Bullard began his appointment in January of 2020, a week before our visit. At this time, neither of the two Associate Dean positions had been filled. -

Board Member Profiles 2021
PALOS VERDES PENINSULA LAND CONSERVANCY BOARD MEMBER PROFILES 2021 OFFICERS Carolynn Petru Occupation: City Administrator/Urban Planner – Retired 2015 President Community Activities: Peninsula Village, Board of Directors and Volunteer Awards & Distinctions: BS Environmental Planning, University of California, Davis; MA Urban Planning, University of California, Los Angeles; American Institute of Certified Planners (1996 – 2015) Rob Kautz Occupation: Principal, HollowayKautz Investments LLC Vice President Community Activities: Los Angeles Police Foundation, Vice Chair 2010-2011, Finance Finance Chair/Treasurer 2002-2010; East Los Angeles College Finance Club speaker/advisor, 2010; Haverford College Alumni Executive Committee 2006-2008; GE Capital Restaurant Leadership Forum, Advisory Board Member 2001-2005; volunteer lecturer on leadership and entrepreneurship, UCLA and Haverford College Awards & Distinctions: LA Business Journal CFO of the Year Finalist, 2010; Robert Half CFO Panel, 2006; National Association for Strategic Planning, Keynote Speaker 2003; National YPO Food and Beverage Roundtable, Keynote Speaker 2003; UCLA Extension/CA Restaurant Association panel 2002; GE Capital’s Innovative Concept of the Year, Award Recipient and Speaker 2002; MBA, Harvard Business School; BA, Economics Diana Bailey Occupation: Attorney – Retired 2015 Secretary Community Activities: Pro Bono, Public Counsel; Volunteer and Member of White Point Community Group and Home Tour, PVPLC; Board of Directors and Volunteer, National Charity League; Executive Board, PTA Rick Wallace Occupation: Certified Public Accountant Treasurer Community Activities: White Point Community Group; LA Biomed Finance Committee; City of Rancho Palos Verdes Finance Advisory Committee 2000-2006; South Bay Lacrosse Association Co-Founder/Chief Financial Officer 2002- 2010; Boy Scout Troop 783 Assistant Scoutmaster 1999-2002 PALOS VERDES PENINSULA LAND CONSERVANCY BOARD MEMBER PROFILES 2021 DIRECTORS Bill Ailor Occupation: Aerospace Fellow, The Aerospace Corp. -

Download Brochure
it begins with a healthy MEDICAL BREAKTHROUGHS AT UCLA UCLA Medical Center opens The first open-heart surgery in the UCLA researchers develop the fi rst western United States is performed techniques for fetal monitoring. at UCLA Medical Center. 1955 1956 1958 UCLA HEALTH UCLA Health. It’s a partnership among you and our doctors, researchers, nurses and staff, who are dedicated to making a difference in your life on a daily basis — all working together to advance the field of medicine, while offering you the finest personal care and treatments available today. People seek us out at the most vulnerable times of their lives. That is why, with our foundation of outstanding academic medicine, cutting-edge technologies and first-rate facilities in Westwood and Santa Monica, we pledge to the 2.5-million patients who enter our medical offices and the more than 100,000 patients who are admitted to our hospitals each year that we will treat each and every one as we would members of our own families. UCLA Health. It begins with you. UCLA Health: Accolades and Achievements Ronald Reagan UCLA Medical Center ranks as the No. 1 UCLA Medical Center, Santa Monica is named a medical center in the western U.S. and No. 5 in the nation distinguished hospital for clinical excellence Mattel Children’s Hospital UCLA ranks among the top UCLA Medical Group awarded Gold Level Achievement U.S. pediatric hospitals for clinical quality by the California Department of Managed Health Care Stewart and Lynda Resnick Neuropsychiatric Hospital at UCLA ranks No. 1 in the West and among the UCLA is a national leader in organ transplantation top-10 in the country UCLA Jonsson Comprehensive Cancer Center is Ophthalmology services at UCLA Stein Eye and Doheny designated as one of only 40 U.S. -

Press Kit (Pdf)
IMAGES IMAGES PROJECT DESCRIPTION The new UCLA Health Training Center is currently in construction in El Segundo, CA. The new complex will house the business and basketball operations as well as a full NBA training center with an exhibition court and arena seating. The new 120,000 square-foot facility is both striking and noteworthy. The project was designed by noted Detroit-based sports and entertainment architect ROSSETTI in conjunction with the Los Angeles architectural office of Perkins + Will. Highlights of the building design include a Sponsor’s Gallery which will be accessible to the public, separate office and game-day entries, separate and secure player parking and entry, and an employee hub/internet café. The new building is specifically designed to facilitate high level training. The state-of-the-art facility is all encompassing for the athlete. The 80,000 square-foot first floor includes a double court gymnasium with on-court smart board and video displays for strategic planning and playbacks; plyometric training areas; weight and conditioning gym that opens to the court; a video theater/screening room directly adjacent to the player lounge; an indoor-outdoor lounge fully outfitted for video, sound and a myriad of digital connections; a barber shop; a player kitchen and training table (as well as an adjacent commercial kitchen directed by a nutritionist); a player quiet room outfitted with blue light spectrum lighting; a state-of-the- art training room with multiple whirlpools, two plunge pools, a resistance pool and cryogenics chamber; a separate training area for the D-Fenders Development League team and all the necessary accessory spaces to support a high level Training, Recovery and Rest program. -
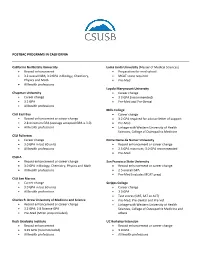
Postbac Programs in California
POSTBAC PROGRAMS IN CALIFORNIA California Northstate University Loma Linda University (Master of Medical Sciences) • Record enhancement • Preparation for med school • 3.2 overall GPA; 3.0 GPA in Biology, Chemistry, • MCAT score required Physics and Math • Pre-Med • All health professions Loyola Marymount University Chapman University • Career change • Career change • 3.3 GPA (recommended) • 3.2 GPA • Pre-Med and Pre-Dental • All health professions Mills College CSU East Bay • Career change • Record enhancement or career change • 3.2 GPA required for advisor letter of support • 2.8 minimum GPA (average accepted GPA is 3.0) • Pre-Med • All health professions • Linkage with Western University of Health Sciences, College of Osteopathic Medicine CSU Fullerton • Career change Notre Dame de Namur University • 3.0 GPA in last 60 units • Record enhancement or career change • All health professions • 2.5 GPA minimum; 3.0 GPA recommended • Pre-Med CSULA • Record enhancement or career change San Francisco State University • 3.0 GPA in Biology, Chemistry, Physics and Math • Record enhancement or career change • All health professions • 2.5 overall GPA • Pre-Med (includes MCAT prep) CSU San Marcos • Career change Scripps College • 3.0 GPA in last 60 units • Career change • All health professions • 3.0 GPA • Test scores (GRE, SAT or ACT) Charles R. Drew University of Medicine and Science • Pre-Med, Pre-Dental and Pre-Vet • Record enhancement or career change • Linkage with Western University of Health • 3.2 GPA; 2.8 Science GPA Sciences, College of -

Cathy A. Sandeen, Phd, Mba
CATHY A. SANDEEN, PHD, MBA CURRENT POSITION Chancellor, University of Wisconsin Colleges and University of Wisconsin- Extension EDUCATION University of California Los Angeles MBA ManageMent 2005 University of Utah PhD ComMunication 1992 San Francisco State University MA Broadcast ComMunication 1981 HuMboldt State University BA Speech Pathology, Summa cum laude 1976 Additional Education/Certifications American Council on Education ACE Fellows Leadership PrograM 2011 UCLA Anderson School of ManageMent Corporate Director Certification 2010 EMPLOYMENT University of Wisconsin 2014-present Chancellor, University of Wisconsin Colleges and University of Wisconsin-Extension Scope and responsibility: Oversees the academic, financial, outreach, and adMinistrative functions of two statewide higher education institutions. UW Colleges encoMpasses 13 two-year transfer colleges and UW Colleges Online, coMprising the third largest institution in the University of Wisconsin System. UW-Extension includes Continuing Education, Outreach and E-Learning, Cooperative Extension, Division of Entrepreneurship and EconoMic DevelopMent, Wisconsin Public Radio, Wisconsin Public Television, Instructional CoMMunications SysteMs and Extension Conference Centers. The combined institutions eMploy 2,200 faculty and staff and have an annual operating budget of $350 Million. These institutions embody the “Wisconsin Idea,” providing access to education, research, and inforMation to all corners of the state. Key accoMplishMents: Oversaw two Major reorganizations within the UW Colleges and UW Cooperative Extension that streaMlined structure and operations and achieved over $8 Million in annual savings. American Council on Education 2012 - 2014 Vice President, Education Attainment and Innovation Scope and responsibility: Oversee ACE’s national agenda to increase postsecondary educational attainMent in the US, including coordination aMong national and international leaders froM across higher education, foundations, business, and governMent. -

Annual Report Fiscal Year 2018-2019
Annual FISCAL YEAR Report 2018 - 2019 DEAN'S MESSAGE EDUCATION TABLE OF CONTENTS r1 Passion for Dental Education Transcends a 40-Year Career Dr. Carol Bibb ’78, professor of oral medicine and orofacial pain, stepped down at the end of the fiscal year after dedicating 40 years to the UCLA School of Dentistry. She was the associate dean for student affairs for 14 years and prior to this important role, she served as the general clinic director for five years. Throughout her tenure, Dr. Bibb taught in a range of formats, from lectures and small group case-based seminars, to preclinical laboratory instruction and chair-side clinical supervision. She has taught pre-doctoral students and residents and has served as a research mentor for dental students and master’s degree candidates. She is now on recall and will focus her energy on curriculum innovations. AT A GLANCE: Dear UCLA School of Dentistry Community, Class of 2023 23 Before we look towards the future, I would like and foundations. We also established our Avg. Dental to highlight our collective accomplishments twelfth endowed chair thanks to a million-dollar 3.77 Admission Test Score during the 2018 – 2019 fiscal year. Here are a gift from Dr. Naomi Ellison ʼ81 and her husband, Overall GPA few of the more important figures that stand Mr. Jim Ellison. 23 GPA+x out. We awarded $4.3M worth of financial aid Avg. of Of course, none of this would be possible were x Total Science to dozens of deserving dental students; we x it not for the hard work and dedication of our x received more than $22M worth of contracts staff, faculty, students, residents, trainees, 3.73 22 and grants in support of our research projects; Science GPA Avg. -

Summer 2021 Magazine
Summer 2021 The Changing Face of Dentistry DEAN'S MESSAGE TABLE of CONTENTS Dental students, Benit Meyer (left) and Naseem Musharbash (right), pose for a photo with Dean Paul Krebsbach to record the UCLA ASDA chapter winning a wellness 1 7 competition last fall. (Photo: Brian Lozano.) 1 Embracing Diversity - One Dental Student at a Time Dental school sets a plan to recruit more students of color 7 Welcome to the Team In it Together Two new faculty members bring their expertise and skills to Oral and Maxillofacial Surgery I WOULD LIKE TO TAKE THIS OPPORTUNITY TO gone on to build successful practices and careers, and they CONGRATULATE THE RESILIENT CLASS OF 2021 reflect on the importance of giving back to the school and 11 What goes well with tacos? Toothbrushes! who graduated on Saturday, May 15, at a small ceremony on the profession. Students and faculty find ways to promote oral health in campus. Fortunately, we were able to safely acknowledge our We take a look back at the celebrated career of Dr. Philip the community despite the pandemic graduates’ accomplishments in an outside venue that somewhat Trask, a pediatric dentist, who has worked for UCLA for just resembled our traditional ceremony. When these graduates over 50 years. He retires at the end of June but will stay on as 15 Alumni Spotlights 11 reflect on this chapter of their lives, I hope that they will take a volunteer faculty member. We can’t thank him enough for his Two alumni find fulfillment and joy in giving back to the with them the skills they’ve gained during the COVID - 19 service and dedication. -
THE BRIDGING the GAP Summer 2020 / Vol 16 / Issue 1
Summer 2020 / Vol 16 / Issue 1 HOOL OF DEN SC TI A ST CL R U Y DiastemaTHE BRIDGING THE GAP 1 The Diastema Summer 2020 / Vol 16 / Issue 1 The Diastema Summer 2020 / Vol 16 / Issue 1 2 Table of Contents Meet the 2020-2021 ASDA Executive Cabinet 03 Coming Full Circle: Dr. Prestonʼs Path to Teaching at UCLA 04 By Ryan Needle ʻ21 2020 ASDA Conference Magic 05 By Kelsey Lomen ʻ22 UCLA ASDA Community Service: A Review of 2019-2020 07 By Lewis Luo ʻ22 A Shift in Focus: Connecting with Pre-Dental Students during A NOTE FROM THE EDITORS COVID-19 08 By Alyssa Janssen ʻ21 Dear Readers, Dental Student Experience During Quarantine 09 Welcome back Bruins, we hope you had a restful break! Our names are Guiselle Murillo By Fernanda Silva Celaya ʻ22 and Stephanie Peacock and we would like to introduce ourselves as the new 2020-2021 Editors-in-Chief of The Diastema. After a short hiatus, we are more excited than ever to Diastem-Art Gallery 11 bring our award-winning newsletter back to life. We hope this issue acts as a way to tie By Ksenia Bubukina ʻ22 everyone back together and allows everyone to, in a way, catch up after a quarter of being apart. There are many interesting, uplifting, and enjoyable articles in store for you. Giving Special Needs Populations a Voice 13 By Stephanie Peacock ʻ22 In this issue, we introduce our new ASDA cabinet, feature several of our noteworthy faculty members, give you a sneak peak into the magic of ASDA conferences, From April 2004 to April 2020: The Evolution of Dental demonstrate how our school relentlessly strove through the COVID-19 crisis in Spring, Licensure 15 highlight the successes of some of our ASDA committees and rising clubs, spotlight our By Berta Taverdi ʻ21 artistically talented peers, and detail the evolution of dental licensure. -

Restorative Dentistry & Endodontics
pISSN 2234-7658 Vol. 44 · Supplement · November 2019 eISSN 2234-7666 November 8–10, 2019 · Coex, Seoul, Korea Restorative DentistryRestorative & Endodontics Restorative Dentistry & Endodontics Vol. 44 Vol. · Supplement Supplement · November 2019 November The Korean Academy of Conservative Dentistry Academy The Korean The Korean Academy of Conservative Dentistry www.rde.ac Vol. 44 · Supplement · November 2019 Restorative Dentistry & Endodontics November 8–10, 2019 · Coex, Seoul, Korea pISSN: 2234-7658 eISSN: 2234-7666 Aims and Scope Distribution Restorative Dentistry and Endodontics (Restor Dent Endod) is a Restor Dent Endod is not for sale, but is distributed to members peer reviewed and open-access electronic journal providing up- of Korean Academy of Conservative Dentistry and relevant to-date information regarding the research and developments researchers and institutions world-widely on the last day of on new knowledge and innovations pertinent to the field of February, May, August, and November of each year. Full text PDF contemporary clinical operative dentistry, restorative dentistry, files are also available at the official website (https://www.rde. and endodontics. In the field of operative and restorative ac; http://www.kacd.or.kr), KoreaMed Synapse (https://synapse. dentistry, the journal deals with diagnosis, treatment planning, koreamed.org), and PubMed Central. To report a change of treatment concepts and techniques, adhesive dentistry, esthetic mailing address or for further information contact the academy dentistry, tooth whitening, dental materials and implant office through the editorial office listed below. restoration. In the field of endodontics, the journal deals with a variety of topics such as etiology of periapical lesions, outcome Open Access of endodontic treatment, surgical endodontics including Article published in this journal is available free in both print replantation, transplantation and implantation, dental trauma, and electronic form at https://www.rde.ac, https://synapse. -

HS 9451 Use of Electronic Information by UCLA Health Workforce
Current Status: Active PolicyStat ID: 8338395 Effective Date: 04/2005 Approved Date: 08/2020 Revised Date: 08/2020 Next Review: 08/2023 Owner: Liangzhou Chen: Mgr Policy Area: Compliance Reference Tags: Lippincott Applicability: Ronald Reagan, Resnick, Santa Monica, Ambulatory Care Use of Electronic Information by UCLA Health Workforce (Employees), HS 9451 PURPOSE This policy sets forth guidelines for the use of Electronic Information Resources by the Workforce. DEFINITIONS Electronic Information Resources" includes, but is not limited to, computer equipment (servers, workstations, laptops and other portable computers), online services, medical devices, mobile devices (tablets, smart phones, digital cameras, etc.), applications (Electronic Health Record, email, databases, other software), storage media (USB drives, DVDs, CDs, magnetic tape, memory cards), and networks. "Protected Health Information" or "PHI" is any individually identifiable health information, in any form or media, whether electronic, paper or oral. "Individually identifiable" means that the health or medical information includes or contains any element of personal identifying information sufficient to allow identification of the individual,COPY such as the patient's name, address, electronic mail address, telephone number, or social security number, genetic or other information that, alone or in combination with other publicly available information, reveals the individual's identity. PHI includes: medical information; patient billing and health insurance information and applies to a patient's past, current or future physical or mental health or treatment. "Electronic Protected Health Information" or "ePHI" is PHI that is transmitted by electronic media or is maintained in electronic media. For example, ePHI includes all data that may be transmitted over the Internet, or stored on a computer, a CD, a disk, magnetic tape or other media.