DEFORMATION and FRACTURE – LAB COURSE the Creep Test
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10-1 CHAPTER 10 DEFORMATION 10.1 Stress-Strain Diagrams And
EN380 Naval Materials Science and Engineering Course Notes, U.S. Naval Academy CHAPTER 10 DEFORMATION 10.1 Stress-Strain Diagrams and Material Behavior 10.2 Material Characteristics 10.3 Elastic-Plastic Response of Metals 10.4 True stress and strain measures 10.5 Yielding of a Ductile Metal under a General Stress State - Mises Yield Condition. 10.6 Maximum shear stress condition 10.7 Creep Consider the bar in figure 1 subjected to a simple tension loading F. Figure 1: Bar in Tension Engineering Stress () is the quotient of load (F) and area (A). The units of stress are normally pounds per square inch (psi). = F A where: is the stress (psi) F is the force that is loading the object (lb) A is the cross sectional area of the object (in2) When stress is applied to a material, the material will deform. Elongation is defined as the difference between loaded and unloaded length ∆푙 = L - Lo where: ∆푙 is the elongation (ft) L is the loaded length of the cable (ft) Lo is the unloaded (original) length of the cable (ft) 10-1 EN380 Naval Materials Science and Engineering Course Notes, U.S. Naval Academy Strain is the concept used to compare the elongation of a material to its original, undeformed length. Strain () is the quotient of elongation (e) and original length (L0). Engineering Strain has no units but is often given the units of in/in or ft/ft. ∆푙 휀 = 퐿 where: is the strain in the cable (ft/ft) ∆푙 is the elongation (ft) Lo is the unloaded (original) length of the cable (ft) Example Find the strain in a 75 foot cable experiencing an elongation of one inch. -

Engineering Viscoelasticity
ENGINEERING VISCOELASTICITY David Roylance Department of Materials Science and Engineering Massachusetts Institute of Technology Cambridge, MA 02139 October 24, 2001 1 Introduction This document is intended to outline an important aspect of the mechanical response of polymers and polymer-matrix composites: the field of linear viscoelasticity. The topics included here are aimed at providing an instructional introduction to this large and elegant subject, and should not be taken as a thorough or comprehensive treatment. The references appearing either as footnotes to the text or listed separately at the end of the notes should be consulted for more thorough coverage. Viscoelastic response is often used as a probe in polymer science, since it is sensitive to the material’s chemistry and microstructure. The concepts and techniques presented here are important for this purpose, but the principal objective of this document is to demonstrate how linear viscoelasticity can be incorporated into the general theory of mechanics of materials, so that structures containing viscoelastic components can be designed and analyzed. While not all polymers are viscoelastic to any important practical extent, and even fewer are linearly viscoelastic1, this theory provides a usable engineering approximation for many applications in polymer and composites engineering. Even in instances requiring more elaborate treatments, the linear viscoelastic theory is a useful starting point. 2 Molecular Mechanisms When subjected to an applied stress, polymers may deform by either or both of two fundamen- tally different atomistic mechanisms. The lengths and angles of the chemical bonds connecting the atoms may distort, moving the atoms to new positions of greater internal energy. -

Oversimplified Viscoelasticity
Oversimplified Viscoelasticity THE MAXWELL MODEL At time t = 0, suddenly deform to constant displacement Xo. The force F is the same in the spring and the dashpot. F = KeXe = Kv(dXv/dt) (1-20) Xe is the displacement of the spring Xv is the displacement of the dashpot Ke is the linear spring constant (ratio of force and displacement, units N/m) Kv is the linear dashpot constant (ratio of force and velocity, units Ns/m) The total displacement Xo is the sum of the two displacements (Xo is independent of time) Xo = Xe + Xv (1-21) 1 Oversimplified Viscoelasticity THE MAXWELL MODEL (p. 2) Thus: Ke(Xo − Xv) = Kv(dXv/dt) with B. C. Xv = 0 at t = 0 (1-22) (Ke/Kv)dt = dXv/(Xo − Xv) Integrate: (Ke/Kv)t = − ln(Xo − Xv) + C Apply B. C.: Xv = 0 at t = 0 means C = ln(Xo) −(Ke/Kv)t = ln[(Xo − Xv)/Xo] (Xo − Xv)/Xo = exp(−Ket/Kv) Thus: F (t) = KeXo exp(−Ket/Kv) (1-23) The force from our constant stretch experiment decays exponentially with time in the Maxwell Model. The relaxation time is λ ≡ Kv/Ke (units s) The force drops to 1/e of its initial value at the relaxation time λ. Initially the force is F (0) = KeXo, the force in the spring, but eventually the force decays to zero F (∞) = 0. 2 Oversimplified Viscoelasticity THE MAXWELL MODEL (p. 3) Constant Area A means stress σ(t) = F (t)/A σ(0) ≡ σ0 = KeXo/A Maxwell Model Stress Relaxation: σ(t) = σ0 exp(−t/λ) Figure 1: Stress Relaxation of a Maxwell Element 3 Oversimplified Viscoelasticity THE MAXWELL MODEL (p. -

Creep-Fatigue Interaction Cumulative Damage
r ^«M!«je^. ^\~*t. re» .• «J^* -•^*'*5i "^' ^•-^^•fB^^^^'C^- '•'• "^ ORNL-4757 '•«* CREEP-FATIGUE INTERACTION and CUMULATIVE DAMAGE EVALUATIONS for TYPE 304 STAINLESS STEEL Hold-Time Fatigue Test Program and Review of Multiaxial Fatigue E. P. Esztergar BLANK PAGE Printed in the United States of America. Available from National Technical Information Service US. Department of Commerce 5285 Port Royal Road. Springfield. Virginia 22151 Price: Printed Copy $3.00; Microfiche $0.95 This report was prepared as an account of work sponsored by the United States Government. Neither the United States nor the United States Atomic Energy Commission, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or impliad, or assumes any legal liability or responsibility for the accuracy, completeness or usefulness of any information, apparatus, product or process drsdosed. or leuiesenu that its use would not infringe privately owned rights. 0RHL-VT5T UC-60 — Reactor Technology Contract No. W-74Q5-eng-26 Reactor Division CHEEP-FATIGUE IHTERACTIOH AM) CUMULATIVE BAM/UJE EVALUATIOHS FOR TYPE 304 STAIHEESS STEEL Hold-Tine Fatigue Test Program and Review of MultlaxLal Fatigue E. P. Esztergar NOTICE— Consultant JUNE 1972 OAK RIDGE HATIOHAL LABORATORY Oak Ridge, Tennessee 37830 operated by UKOH CAREER CORPORATIOH for the U.S. ATOMIC EHERGY COMMISSIOR tmawmm of nw Mctmnr n mumm iii CONTENTS Page PREFACE . v ACKNOWLEDGMENTS vii ABSTRACT 1 1. INTRODUCTION 2 2. BACKGROUND k 2.1 Review of Time Effects on Fatigue Behavior k Strain rate k Cyclic relaxation U Cyclic creep 9 2.2 Basis for High-Temperature Design Procedures 10 The t-n diagram 12 Continuous-cycle fatigue data 15 Cyclic-relaxation data 18 Cyclic-creep data 21 3. -

Creep-Fatigue Failure Diagnosis
Review Creep-Fatigue Failure Diagnosis Stuart Holdsworth Received: 22 October 2015 ; Accepted: 6 November 2015 ; Published: 16 November 2015 Academic Editor: Robert Lancaster EMPA: Swiss Federal Laboratories for Materials Science and Technology Überlandstrasse 129, Dübendorf CH-8600, Switzerland; [email protected]; Tel.: +41-58-765-47-32 Abstract: Failure diagnosis invariably involves consideration of both associated material condition and the results of a mechanical analysis of prior operating history. This Review focuses on these aspects with particular reference to creep-fatigue failure diagnosis. Creep-fatigue cracking can be due to a spectrum of loading conditions ranging from pure cyclic to mainly steady loading with infrequent off-load transients. These require a range of mechanical analysis approaches, a number of which are reviewed. The microstructural information revealing material condition can vary with alloy class. In practice, the detail of the consequent cracking mechanism(s) can be camouflaged by oxidation at high temperatures, although the presence of oxide on fracture surfaces can be used to date events leading to failure. Routine laboratory specimen post-test examination is strongly recommended to characterise the detail of deformation and damage accumulation under known and well-controlled loading conditions to improve the effectiveness and efficiency of failure diagnosis. Keywords: failure diagnosis; creep-fatigue; material condition; mechanical analysis 1. Introduction The diagnosis of failures invariably involves consideration of both the associated material condition and the results of a mechanical analysis of prior operating history. Material condition refers not only to a knowledge of the chemical composition and mechanical properties relative to those originally specified for the failed component, but also the appearance and extent of microstructural and physical damage responsible for failure. -

Stress Corrosion Cracking, Fatigue-Creep Damage, Hydrogen Embrittlement, Stress-Assisted Diffusion, Stress Concentration, Lifetime
International Journal of Mechanics and Applications 2014, 4(2): 50-57 DOI: 10.5923/j.mechanics.20140402.03 Lifetime Estimation of Chain Plates for Floodgates Working in Seawater by Continuum Fatigue-Creep Damage Theory and Transient Analysis of Diffusion Process of Hydrogen Hak Jin Song*, Sun Jong Jon, Gi Chol Yang, Nam Chol Kim Department of Mechanics of Materials, Kim Chaek University of Technology, Pyongyang, Korea Abstract Numerical estimation of lifetime of chain plates made of 45Cr high strength steel for floodgates working in seawater has been performed by considering fatigue-creep crack initiation and hydrogen assisted crack propagation at the plate. The computational model has been validated by some related experiments on the material. First, tensile test results for the material of chain plates used for 20 years in seawater were compared with the ones for new material. According to the results, yield stress and fracture ductility of the material used for 20 years in seawater are considerably lower than the ones of new material. Second, records of operations of the floodgates were analyzed to estimate real lifetime of the chain plates and some other parameters which are necessary for computational study on the same objects. According to the records of operating history of the floodgates, average lifetime of the chain plates falls within the limit of 18~22 years. Third, lifetime of the chain plates was estimated by continuum fatigue-creep damage theory and transient analysis of the diffusion process of atomic hydrogen in the metallic lattice. Estimated lifetime of the chain plates was a little longer than the real lifetime obtained from the records because of some unexpected conditions. -

Investigation Into the Effects of Operating Conditions and Design
Mechanics and Mechanical Engineering Vol. 15, No. 3 (2011) 237{247 ⃝c Technical University of Lodz Investigation into the Effects of Operating Conditions and Design Parameters on the Creep Life of High Pressure Turbine Blades in a Stationary Gas Turbine Engine Samir Eshati School of Engineering Cranfield University Cranfield, Bedford MK43 0AL, UK samir.eshati@cranfield.ac.uk Abdullahi Abu Panagiotis Laskaridis Anthony Haslam Cranfield University a.abu@cranfield.ac.uk UK sP.Laskaridis@cranfield.ac.uk A.Haslam@cranfield.ac.uk Received (21 August 2011) Revised (20 September 2011) Accepted (28 September 2011) A physics{based model is used to investigate the relationship between operating condi- tions and design parameters on the creep life of a stationary gas turbine high pressure turbine (HPT) blade. A performance model is used to size the blade and to determine its stresses. The effects of radial temperature distortion, turbine inlet temperature, ambient temperature and compressor degradation on creep life are then examined. The results show variations in creep life and failure location along the span of the blade enabling better informed design and maintenance decisions. Keywords: Lifing, creep, turbine, blade, performance, degradation 1. Introduction Gas turbines are required to operate under conditions of high temperature and mechanical loading. At these conditions, the components undergo various time{ dependent degradations that result in failure mechanisms such as low/high cycle fatigue, corrosion/oxidation and creep [10]. Creep significantly reduces the compo- 238 Eshati, S., Abu, A., Laskaridos, P., Haslam, A. nent life of stationary gas turbines. The creep life consumption of a gas turbine depends on the design of the hot section components, duty cycle and the environ- ment in which it operates. -

Compression Creep and Thermal Ratcheting Behavior of High Density Polyethylene (HDPE)
polymers Article Compression Creep and Thermal Ratcheting Behavior of High Density Polyethylene (HDPE) Rahul Palaniappan Kanthabhabha Jeya * ID and Abdel-Hakim Bouzid Department of Mechanical engineering, École de Technologie Supérieure, 1100 Notre Dame O, Montréal, QC H3C1K3, Canada; [email protected] * Correspondence: [email protected] Received: 25 December 2017; Accepted: 2 February 2018; Published: 7 February 2018 Abstract: The characterization of thermal ratcheting behavior of high density polyethylene (HDPE) material coupled with compressive creep is presented. The research explores the adverse influence of thermal cycling on HDPE material properties under the effect of compressive load, number of thermal cycles, creep time period, and thermal ratcheting temperature range. The compressive creep analysis of HDPE shows that the magnitude of creep strain increases with increase in magnitude of applied load and temperature, respectively. The creep strain value increased by 7 and 28 times between least and maximum applied temperature and load conditions, respectively. The creep modulus decreases with increase in compressive load and temperature conditions. The cumulative deformation is evident in the HDPE material, causing a reduction in the thickness of the sample under thermal ratcheting. The loss of thickness increases with increase in the number of thermal cycles, while showing no sign of saturation. The thermal ratcheting strain (TRS) is influenced dominantly by the applied load condition. In addition, the TRS decreases with increase in creep time period, which is cited to the extended damage induced due creep. The results highlight the need for improved design standard with inclusion of thermal ratcheting phenomenon for HDPE structures particularly HDPE bolted flange joint. -
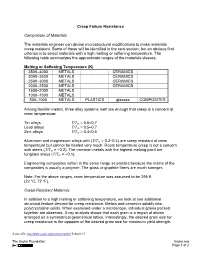
Creep Failure Resistance Comparison of Materials the Materials
Creep Failure Resistance Comparison of Materials The materials engineer can devise microstructural modifications to make materials creep resistant. Some of these will be identified in the next section, but an obvious first criterion is to select materials with a high melting or softening temperature. The following table summarizes the approximate ranges of the materials classes. Melting or Softening Temperature (K) 3500–4000 METALS CERAMICS 3000–3500 METALS CERAMICS 2500–3000 METALS CERAMICS 2000–2500 METALS CERAMICS 1500–2000 METALS 1000–1500 METALS 500–1000 METALS PLASTICS glasses COMPOSITES Among familiar metals, three alloy systems melt low enough that creep is a concern at room temperature: Tin alloys T/Tm = 0.6–0.7 Lead alloys T/Tm = 0.5–0.7 Zinc alloys T/Tm = 0.4–0.5 Aluminum and magnesium alloys with (T/Tm = 0.3–0.4) are creep resistant at room temperature but cannot be heated very much. Room temperature creep is not a concern with steels (T/Tm = ~0.2). The common metals with the highest melting point are tungsten alloys (T/Tm = ~0.1). Engineering composites soften in the same range as plastics because the matrix of the composites is usually a polymer. The glass or graphite fibers are much stronger. Note: For the above ranges, room temperature was assumed to be 295 K (22 ºC, 72 ºF). Creep-Resistant Materials In addition to a high melting or softening temperature, we look at one additional structural feature desired for creep resistance. Metals and ceramics solidify into polycrystalline solids. When examined under a microscope, individual grains packed together are observed. -

Mechanical Failure – Creep
High temperature applications What happen to the strength at elevated temperature -Steel power plants -Oil refineries -Chemical plants Strength becomes……… High operating temperatures very dependent to strain rate and time of Engine jet ----1400 oC exposure Steam turbine power plants: pipes carry steam (~566 oC, pressure ~ 3500 psi) Called Creep surface reentry temperature ~ 2800 oC (Apollo) …Temperatures generated within the hottest area during ballistic reentry may exceed 11,100°C MECHANICAL FAILURE – CREEP ISSUES TO ADDRESS... MECHANICAL FAILURE - • Creep curve CREEP • Revisit dislocations • Revisit diffusion • Creep testing • Creep failure • Larson-Miller parameter WHAT IS CREEP? • Many engineering components are exposed to high temperature for a long period of time. • Changes within the component due to this (at constant Time – dependent permanent plastic deformation, which generally occurs at high temperatures (T > 0.4T ), under a constant load or stress. stress) is called Creep. m • e.g. Turbine blade within a jet engine, steam generator. • It can also happened at room temperature for soft metals such as Lead. • It is a slow process, where deformation changes with time. Creep is important in applications such as: turbine blades (jet engines), gas turbines, power plants (boilers and steam lines) which must operate 800-1000 OC at high stresses and high temperatures without any changes in dimensions. THEORY OF CREEP Creep behaviour of a metal is determined by measuring the strain World trade center, WTC collapsed, due to creep (ε) deformation as function of time under constant stress 21 CREEP • Creep occurs even with high strength materials with high heat resistant. strain, ε • At high temperature atomic bonding starts to fail, causing movement of atoms and atomic planes. -

Behavior of Solids in Simple Mechanical Tests (PDF)
Notes L.6 Behavior of Solids In Simple Mechanical Tests Originated by John E. Meyer and Modified by Mujid S Kazimi Department of Nuclear Science and Engineering Massachusetts Institute of Technology 1 Purpose. The purpose of these notes is to describe in a brief introductory manner: (a) Some simple mechanical tests that are used to characterize the behavior of structural materials; (b) The kinds of physical behavior that is observed during the tests ; and (c) Some of the quantities which are extracted for input to structural mechanics analysis. 2 Tensile Test (Uniaxial) Test Units are given in Parenthesis –SI Units F = Axial force (N) imposed on the test specimen ! = gage length (m) m =Meter = length ! s = second (time) N = Newton = Force Pa = Pascal = Stress or Pressure = N/m2 °C = Celsius Degrees F ! =Distance between fiducial marks on the test specimen A= minimum cross sectional area (m2) of the test specimen o = Subscript denoting the initial unloaded condition f = subscript denoting the final condition of the broken specimen 1 Measurements and Data Reduction - measure ! vs. F while pulling the specimen - Also obtain Af by reassembling the pieces after the specimen has broken - Define strain (“engineering strain” ) to be e, where: e = (!-!O/!O) (1) Define stress (“engineering Stress”) to be S, where: S= F/Ao (2) ( ) -Define reduction of Area, RA, to be: " A ! A % R = $ o f ' (3) A A # o & Material Property Information Obtained - Stress-Strain curve and Derived quantities Fracture E Sy Su Stress, S (Pa) Curve is not to scale 0.002 Strain, e <0.2%> - Derived Quantities - E = Young’s modulus (Pa), slope of the stress- strain curve at low strains -Sy = Yield Stress (Pa), “Stress at 0.2% offset” - Su = Ultimate Stress (Pa), maximum stress reached during the test 2 3 Creep Test: Test Define stress and strain as above. -

Understanding Creep Failure of Plastics Continuous Stress Over Long Periods Leads to Creep Rupture
CONSULTANT’S CORNER Understanding Creep Failure of Plastics Continuous stress over long periods leads to creep rupture By Jeffrey Jansen The Madison Group, Madison, Wisconsin, USA his article addresses one of the most important yet • time and temperature act on plastics in a similar way; least understood plastics failure mechanisms, creep and rupture. Creep rupture occurs within plastic parts • unexpected brittle failure can occur under conditions Tthat are exposed to continuous stress over an extended of low-to-moderate applied stress. period of time ( Figure 1 ). Common design features that expose a plastic product to continuous stress, and thus are at risk of creep rupture, include: • structural components (beams, columns, hanging supports), • hydrostatic pressure vessels (pipes, tanks, valves), and • joints and interference fittings (over- moldings, press fits, snap fits, mechanical fasteners). In considering why creep rupture occurs so frequently, a major contributing cause is the general lack of awareness of the effects of time on polymeric materials, par - ticularly at the design stage. Designing and manufacturing plastic components offers unique challenges compared with other materials, especially metals. In the sections below, readers will gain an understanding of how: • the properties of molded plastics will change over time when under contin - Figure 1: Many different types of design features and service functions result in the uous stress; application of continuous stress. This can produce cracking through creep rupture. 32 | PLASTICS ENGINEERING | JULY / AUGUST 2015 | www.4spe.org | www.plasticsengineering.org Viscoelasticity When a material is placed under a constant stress, the response observed initially will be a function of the stiffness, In order to understand creep and avoid product failure, or modulus, of the material.