Amicus Curiae Brief of 83 Oklahoma Legislators and Americans United for Life Action in Support of the Defendants/Appellants, Filed on Motion for Leave to File Brief
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LEGISLATIVE ISSUES REPORT Legislation, Lobbying Advocacy Jennifer James Mccollum, APR Public Relations & Community Development
LEGISLATIVE ISSUES REPORT Legislation, Lobbying Advocacy Jennifer James McCollum, APR Public Relations & Community Development MAY 2018 BUDGET RECAP 2nd SESSION, 56th LEGISLATURE BUDGET RECAP • HB 1010xx | $420 million revenue-raising bill passed during the special session: • Gross Production tax on oil and gas wells goes up from 2 to 5 percent at a $170 million cost to the industry • Motor fuel will cost $.03 more per gallon • Cigarettes will go up $1 per pack • Will pay for teacher, support staff and state employee raises • HB 1011 | Revenue, Taxation • Prohibits taxpayers from claiming $17,000+ in itemized deductions, raising approximately $94 million a year • Larger Internet sellers, such as Amazon, must now collect and remit sales taxes from third-party vendors, raising approximately $20 million a year 2nd SESSION, 56th LEGISLATURE BUDGET RECAP • HB 1086 | Capital Gains • Failed to Pass; Would have ended Oklahoma’s capital gains deduction, raising $100 million revenue for the state • SB 888 | Wind • Bill to create a new gross production tax on wind energy and eliminate paying out refunds on tax credits failed • HB 1024 | State Employees • State employees will receive their first raise in about a decade. Pay increases will range from $700 to $2,000 • State employees wanted a $7,500 across-the-board increase over three years 2nd SESSION, 56th LEGISLATURE BUDGET RECAP • Public Education: Budget now $2.4 billion • Teachers will receive an average increase of $6,000 starting this fall • Schools will share $52 million for support staff raises, $33 million for textbooks and $17 million for general school funding Source • SB 1115 to reduce class sizes, and SB 1104 to prevent lunch shaming failed REPEAL of HB 1010xx REFERENDUM • Definition: A direct vote in which the electorate votes on a particular proposal. -
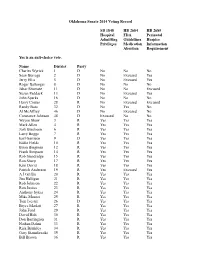
2014 Oklahoma Senate Vote Tracker
Oklahoma Senate 2014 Voting Record SB 1848 HB 2684 HB 2685 Hospital FDA Perinatal Admitting Guidelines Hospice Privileges Medication Information Abortion Requirement Yes is an anti-choice vote. Name District Party Charles Wyrick 1 D No No No Sean Burrage 2 D No Excused Yes Jerry Ellis 5 D No Excused Yes Roger Ballenger 8 D No No No Jabar Shumate 11 D No No Excused Susan Paddack 13 D No Excused Yes John Sparks 16 D No No No Harry Coates 28 R No Excused Excused Randy Bass 32 D No Yes No Al McAffrey 46 D No Excused No Constance Johnson 48 D Excused No No Wayne Shaw 3 R Yes Yes Yes Mark Allen 4 R Yes Yes Yes Josh Brecheen 6 R Yes Yes Yes Larry Boggs 7 R Yes Yes Yes Earl Garrison 9 D Yes Yes Yes Eddie Fields 10 R Yes Yes Yes Brian Bingman 12 R Yes Yes Yes Frank Simpson 14 R Yes Yes Yes Rob Standridge 15 R Yes Yes Yes Ron Sharp 17 R Yes Yes Yes Kim David 18 R Yes Yes Yes Patrick Anderson 19 R Yes Excused Yes A J Griffin 20 R Yes Yes Yes Jim Halligan 21 R Yes Yes Yes Rob Johnson 22 R Yes Yes Yes Ron Justice 23 R Yes Yes Yes Anthony Sykes 24 R Yes Yes Yes Mike Mazzei 25 R Yes Yes Yes Tom Ivester 26 D Yes Yes Yes Bryce Marlatt 27 R Yes Yes Yes John Ford 29 R Yes Yes Yes David Holt 30 R Yes Yes Yes Don Barrington 31 R Yes Yes Yes Nathan Dahm 33 R Yes Yes Yes Rick Brinkley 34 R Yes Yes Yes Gary Stanislawski 35 R Yes Yes Yes Bill Brown 36 R Yes Yes Yes Dan Newberry 37 R Yes Yes Yes Mike Schulz 38 R Yes Yes Yes Brian Crain 39 R Yes Yes Yes Cliff Branan 40 R Yes Yes Yes Clark Jolley 41 R Yes Yes Yes Cliff Aldridge 42 R Excused Yes Excused Corey Brooks 43 R Yes Yes Yes Ralph Shortey 44 R Yes Yes Yes Kyle Loveless 45 R Yes Yes Yes Greg Treat 47 R Yes Yes Yes . -

Name Legislative Body Party District Rep. Casey Murdock House
Name Legislative Body Party District Lobbying Funds Received (Jan. 1-May 31, 2016) Rep. Casey Murdock House Republican 61 $2,972 Rep. Tom Newell House Republican 28 $2,626 Sen. Brian Bingman Senate Republican 12 $2,577 Sen. Mike Schulz Senate Republican 38 $2,449 Rep. Chris Kannady House Republican 91 $2,381 Sen. Kim David Senate Republican 18 $2,309 Rep. John Pfeiffer House Republican 38 $2,294 Rep. Scott Inman House Democrat 94 $2,161 Rep. Josh Cockroft House Republican 27 $2,147 Rep. Jon Echols House Republican 90 $2,125 Rep. Justin Wood House Republican 26 $2,103 Rep. Charles McCall House Republican 22 $2,067 Rep. Glen Mulready House Republican 68 $2,061 Sen. Greg Treat Senate Republican 47 $1,931 Rep. Ben Sherrer House Democrat 8 $1,923 Rep. James Leewright House Republican 29 $1,914 Rep. Scott Biggs House Republican 51 $1,806 Rep. David Derby House Republican 74 $1,773 Rep. Chad Caldwell House Republican 40 $1,758 Rep. Eric Proctor House Democrat 77 $1,748 Rep. Scooter Park House Republican 65 $1,677 Rep. Michael Rogers House Republican 98 $1,655 Rep. Jason DunningtonHouse Democrat 88 $1,629 Rep. Kevin Wallace House Republican 32 $1,627 Sen. Eddie Fields Senate Republican 10 $1,593 Rep. Sean Roberts House Republican 36 $1,545 Sen. Stephanie Bice Senate Republican 22 $1,534 Sen. Gary StanislawskiSenate Republican 35 $1,531 Sen. Jason Smalley Senate Republican 28 $1,481 Rep. John MontgomeryHouse Republican 62 $1,456 Rep. Lee Denney House Republican 33 $1,435 Rep. Doug Cox House Republican 5 $1,414 Rep. -

A Conversation with Secretary of Transportation Gary Ridley
November/December 2016 A Conversation With Secretary of Transportation Gary Ridley towntalk NOVEMBER/DECEMBER 2016 Published Bi-Monthly, By The Oklahoma Municipal Contractors Association P.O. Box 891797, Oklahoma City, Oklahoma 73189 Rick J. Moore, Editor calendar of events Thursday January 19, 2017 OMCA January membership Luncheon Twin Hills Golf & Country Club Oklahoma City, OK - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Tuesday February 14, 2017 Oklahoma City City Council Primary Election Wards 1,3,4 and 7 Oklahoma City, OK - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Thursday February 16, 2017 OMCA February Membership Luncheon Twin Hills Golf & Country Club Oklahoma City, OK - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Thursday March 16, 2017 OMCA March Membership Luncheon Twin Hills Golf & Country Club Oklahoma City, OK - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Tuesday April 4, 2017 Oklahoma City City Council General Election (If needed) Wards 1,3,4 and 7 Oklahoma City, OK - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - Thursday April 20, 2017 OMCA April Membership Luncheon Twin Hills Golf & Country Club Oklahoma City, OK - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - -

Senate Journal Feb 01, 2016
Senate Journal Second Regular Session of the Fifty-fifth Legislature of the State of Oklahoma First Legislative Day, Monday, February 1, 2016 Pursuant to Article V, Section 26, of the Constitution of the State of Oklahoma, the Senate of the Second Regular Session of the Fifty-fifth Legislature assembled in its Chamber at 12:00 noon. The President of the Senate, Lieutenant Governor Todd Lamb, called the Senate to Order. Roll Call: Present: Allen, Anderson, Barrington, Bass, Bice, Bingman, Boggs, Brecheen, Brooks, Brown, Crain, Dahm, David, Dossett, Fields, Floyd, Ford, Fry, Garrison, Griffin, Halligan, Holt, Jech, Jolley, Justice, Marlatt, Matthews, Mazzei, Newberry, Paddack, Pittman, Quinn, Schulz, Sharp, Shaw, Shortey, Silk, Simpson, Smalley, Sparks, Standridge, Stanislawski, Sykes, Thompson, Treat, Wyrick and Yen.—47. Excused: Loveless.—1. President Lamb declared a quorum present. The invocation was offered by Pastor David Player, First United Methodist Church, Altus, the guest of Senator Schulz. INTRODUCTIONS Senator Newberry introduced his wife, Laura, and their children, Claire, Paige, Eva and Alex, and asked unanimous consent, which was granted, that they be named Honorary Senators for this legislative day; President Lamb introduced his wife, Monica; Senator Barrington introduced his wife, Jennifer; Senator Stanislawski introduced his wife, Dayna; and Senator Dossett introduced his parents, Rick and Suzanne Dossett, his Godparents, Danny and Patty O’Shea, his sisters Beverly Arnold and Joanna Dossett, his niece, Dora Barber and his nephew, Hugh Barber, and former Senator Mary Easley, to the Senate. 2 Senate Journal COMMUNICATION RESIGNATION OF SENATOR RICK BRINKLEY August 20, 2015 The Honorable Chris Benge Oklahoma Secretary of State 2300 N. -

Oklahoma's Elected Officials
Oklahoma’s Elected Officials The Oklahoma State Senate There are 48 members in the Oklahoma State Senate. By law, the Lieutenant Governor is president of the Senate, but the role is limited to presiding over special sessions and casting the deciding vote in case of a tie. The law also provides that the Senate will elect a President Pro Tempore, while the House of Representatives elects a Speaker of the House. State senators serve staggered four-year terms. Senators in odd-numbered districts were elected in 2012. Those in even-numbered districts will be elected in 2014. Legislators are paid $38,400 annually, along with certain necessary expenses. The President Pro Tempore of the State Senate and the Speaker of the House of Representatives are paid an additional $17,932 annually, and the majority floor leaders and the minority floor leaders of each House are paid an additional $12,364 per year. The Senate occupies the third, fourth and fifth floors on the east side of the rotunda in the state Capitol. Joint sessions are held in the House Chambers. The Senate Chambers are located on the fourth floor, with the visitors' gallery on the fifth floor. President Pro Tempore Senator Brian Bingman District 12 Bingman was born on December 9, 1953, in Tulsa, OK. He received a BBA in Petroleum Land Management from the University of Oklahoma in 1976. He and his wife Paula, have three children, Annie, Blake and Rebecca. He is currently employed by Uplands Resources Inc. in Tulsa as Vice-President of Land and Operations. Bingman served as Mayor of Sapulpa from 1992 -2004 and served in the House of Representatives for District 30 from 2004-2006. -

Advocate 06-18
In This Issue Former Marlow Mayor Brad Boles Sworn in Today 1 Issue No. 06-18 March 16, 2018 Teacher Pay Raise with Increase in Sales Tax Fails Senate 1 FORMER MARLOW MAYOR BRAD BOLES SWORN IN TODAY Action in Deadline Week 2 Bills on the Move 3 Today, the House of Representatives was made complete when former Marlow Mayor Brad Boles was sworn into as a Representative for District 51. Rep. Boles replaces former Rep. Scott Biggs who left early for a job at the federal level. Boles was elected as Mayor of Marlow in 2014. He was active in the OML, the Congress of Mayors and the Community Leadership Development class. Congratulations Representative Boles. OML is excited to have another municipal voice at the Capitol. Oklahoma Municipal TEACHER PAY RAISE WITH INCREASE IN SALES TAX FAILS SENATE League On Wednesday, the State Senate gutted HB 1033xx in the Second Special OML Advocate is published by the Session and replaced it with language that was part of the revenue plan to raise Oklahoma Municipal League. Forward funding for education. The bill increased the States sales tax rate from 4.5% to your comments or suggestions to: 5.5% ensuring that Oklahoma would have one of the highest average combined sales tax rate in the nation, which will drive many to shop online. Oklahoma Municipal League A trailer bill, SB 861 would have removed the State’s portion of the grocery sales 201 N.E. 23rd Street OKC, OK 73105 tax exemption. However, they stated in the language that the cities and counties Phone: 1-800-324-6651 / 405-528- would have been allowed to collect their portion of the grocery sales taxes. -

Public Policy Guide Banking That Fits Your Needs
2017 GREATER OKLAHOMA CITY CHAMBER PUBLIC POLICY GUIDE BANKING THAT FITS YOUR NEEDS Whether it’s a checking account today, a home loan tomorrow or retirement planning for your future, Arvest Bank has the products and services to t your needs. Convenient locations and extended hours with online and mobile banking give you the features of a large bank with the personal service of a community banker. arvest.com Open an account at your nearest Arvest location today. Member FDIC TABLE OF CONTENTS Message from the Chair ........ page 2 Pro-Business Scorecard .......page 14 Government Relations Staff .. page 3 Elected Officials Directory ...page 16 Greater OKC Chamber PAC .. page 4 Chamber Leadership ............page 34 2017 Public Policy Priorities .. page 5 GOVERNMENT RELATIONS BENEFACTORS 2017 Public Policy Guide 2017 Public Policy GOVERNMENT RELATIONS SPONSORS Enable Midstream Partners Google 1 MESSAGE FROM THE CHAIR The Greater Oklahoma City Chamber takes pride in its role as the voice of business and the visionary organization of Oklahoma City. It is a role that we have held for more than 125 years, and as one of the largest chambers of commerce in the United States, we believe that our organization has an enormous impact in the political arena. As we begin the legislative session, it is a critical time to fully engage in the political process. The state is facing tough budgeting decisions, and many of our most effective economic development programs are up for review. Our education system is at a crossroads. And uncertainty at the federal level leads to questions about heath care and transportation outcomes on a state and local level. -

Primary Election Candidate Survey June 24, 2014
Oklahomans For Life Letter Vol. 39, No. 1 www.OkForLife.org June 2014 Candidate Surveys for June 24, 2014 Primary Election State Candidate Survey days), or when the pregnancy resulted from an act of incest committed against a minor (with the In anticipation of the Primary Election on perpetrator reported to law enforcement authorities)? Tuesday, June 24, 2014, Oklahomans For Life asked the twelve questions below of candidates for Yes____ No____ statewide office and for the Oklahoma state Senate and the Oklahoma state House of 3) Recently, there have been attempts in several Representatives. The most important question is states to pass a state Equal Rights Amendment which No. 1, and the candidates’ answers to that would amend the state Constitution to invalidate any question are written in capital letters. (We asked law or government policy that discriminates “on statewide candidates, who cannot literally “vote account of sex.” In some of the states that have for” these measures, to indicate whether they already added this or similar provisions to their state “support” them.) For state House and Senate constitutions, courts have used them to invalidate races, the counties or partial counties (marked by limits on abortion. The New Mexico Supreme Court, an asterisk) in the district are indicated after the e.g., in 1998 unanimously ruled that the New Mexico district number. The initial after a candidate’s ERA required state funding of abortion. We oppose name represents party affiliation (Republican, the ERA unless an “abortion neutralization” Democrat, or Independent). On each question, a amendment is added to ensure that the ERA will not “yes” answer indicates agreement with the change abortion policy in any way. -

Senatorsonsocialmedia Page 1 Senator Age Twitter
SenatorsOnSocialMedia Senator Age Twitter Facebook Notes Mark Allen (R) District 4 67 N/A https://www.facebook.com/SenatorMarkAllen Stephanie Bice (R) District 22 42 @stephaniebice https://www.facebook.com/sibice Corey Brooks (R) District 43 36 @SenCoreyBrooks https://www.facebook.com/SenatorCoreyBrooks Kim David (R) District 18 54 @kimdavid2010 https://www.facebook.com/kimdavidforstatesenate John Ford (R) District 29 70 N/A https://www.facebook.com/john.w.ford.50 Jim Halligan (R) District 21 79 N/A N/A Ron Justice (R) District 23 70 N/A N/A Mike Mazzei (R) District 25 50 N/A https://www.facebook.com/mike.mazzei.7 Marty Quinn (R) District 2 43 N/A https://www.facebook.com/marty.quinn.9 Ralph Shortey (R) District 44 34 N/A https://www.facebook.com/rshortey John Sparks (D) District 16 47 @SenatorSparks https://www.facebook.com/John-Sparks-28054381045/ Roger Thompson (R) District 8 58 N/A https://www.facebook.com/roger.thompson.16 Patrick Anderson (R) District 19 47 N/A N/A Brian Bingman (R) District 12 62 @oksenategop https://www.facebook.com/brian.bingman.7 Private Facebook Bill Brown (R) District 36 71 N/A N/A J.J. Dossett (D) District 34 31 @DossettFor34 https://www.facebook.com/dossett4senate Jack Fry (R) District 42 65 N/A https://www.facebook.com/jack.fry.520 David Holt (R) District 30 37 @davidfholt https://www.facebook.com/davidfullerholt Kyle Loveless (R) District 45 42 @kyledloveless https://www.facebook.com/LovelessforSenate/ Dan Newberry (R) District 37 40 @sendannewberry https://www.facebook.com/Senator-Dan-Newberry-138475611252/ -

Oklahoma State Senate Senate Leadership
99 Oklahoma State Senate Senate Leadership President Lt. Gov. Todd Lamb Minority Leader Andrew Rice President Pro Tempore Brian Bingman Asst. Min. Floor Leader Sean Burrage Majority Floor Leader Mike Schulz Asst. Min. Floor Leader Roger Ballenger Assistant Floor Leader Anthony Sykes Asst. Min. Floor Leader John Sparks Assistant Floor Leader Clark Jolley Asst. Min. Floor Leader Charles Wyrick Assistant Floor Leader John Ford Min. Whip Earl Garrison Majority Whip Cliff Branan Min. Whip Judy E. McIntyre Majority Whip Dan Newberry Min. Whip Susan Paddack Majority Whip Gary Stanislawski Min. Caucus Chair Tom Ivester Majority Whip Rob Johnson Caucus Chair Bryce Marlatt State Senators by District This list of senators by district is given as a cross-reference. In the section following, senators’ names are arranged in alphabetical order. Dist. Name Dist. Name Dist. Name 1 Charles Wyrick (D) 17 Charles Laster (D) 33 Tom Adelson (D) 2 Sean Burrage (D) 18 Kim David (R) 34 Rick Brinkley (R) 3 Jim Wilson (D) 19 Patrick Anderson (R) 35 Gary Stanislawski (R) 4 Mark Allen (R) 20 David Myers (R) 36 Bill Brown (R) 5 Jerry Ellis (D) 21 Jim Halligan (R) 37 Dan Newberry (R) 6 Josh Brecheen (R) 22 Rob Johnson (R) 38 Mike Schulz (R) 7 Richard Lerblance (D) 23 Ron Justice (R) 39 Brian Crain (R) 8 Roger Ballenger (D) 24 Anthony Sykes (R) 40 Cliff Branan (R) 9 Earl Garrison (D) 25 Mike Mazzei (R) 41 Clark Jolley (R) 10 Eddie Fields (R) 26 Tom Ivester (D) 42 Cliff Aldridge (R) 11 Judy Eason McIntyre (D) 27 Bryce Marlatt (R) 43 Jim Reynolds (R) 12 Brian Bingman (R) 28 Harry Coates (R) 44 Ralph Shortey (R) 13 Susan Paddack (D) 29 John Ford (R) 45 Steve Russell (R) 14 Frank Simpson (R) 30 David Holt (R) 46 Andrew Rice (D) 15 Jonathan Nichols (R) 31 Don Barrington (R) 47 Greg Treat (R) 16 John Sparks (D) 32 Randy Bass (D) 48 Constance Johnson (D). -

Senate Committees
126 Senate Committees Chairs and Vice Chairs of committees are listed first. Senate President Pro Tempore Brian Bingman is ex-officio voting member of all senate committees. Agriculture and Rural Development—Eddie Fields, Ron Justice, Mark Allen, Patrick Anderson, Don Barrington, Randy Bass, Jerry Ellis, Tom Ivester, David Myers, Frank Simpson, Anthony Sykes, and Charles Wyrick. Appropriations—David Myers, Clark Jolley, Roger Ballenger, Cliff Branan, Rick Brinkley, Sean Burrage, Brian Crain, John Ford, Jim Halligan, Tom Ivester, Ron Justice, Bryce Marlatt, Dan Newberry, Jonathan Nichols, Susan Paddack, Andrew Rice, Gary Stani- slawski, Anthony Sykes, Jim Wilson, and Charles Wyrick. Appropriations Subcommittees— All subcommittee members are members of the standing Appropriations Committee. The chair of the Appro- priations Committee is an ex officio and voting member of each subcommittee. Education—Jim Halligan, John Ford, Cliff Aldridge, Josh Brecheen, Rick Brinkley, Judy Eason McIntyre, Earl Garrison, Mike Mazzei, Susan Paddack, Frank Simpson, John Sparks, and Gary Stanislawski. General Government and Transportation—Bryce Marlatt, Cliff Branan, Randy Bass, Bill Brown, Sean Burrage, Harry Coates, Anthony Sykes, and Charles Wyrick. Health and Human Services—Clark Jolley, Kim David, Tom Adelson, Mark Allen, David Holt, Constance Johnson, Dan Newberry, Greg Treat, and Jim Wilson. Natural Resources and Regulatory Services—Ron Justice; Eddie Fields, Roger Ballenger, Brian Crain, Jerry Ellis, and Rob Johnson. Public Safety and Judiciary—Jonathan Nichols, Don Barrington, Tom Ivester, Charlie Laster, Richard Lerblance, Jim Reynolds, Steve Russell, and Ralph Shortey. Business and Commerce—Dan Newberry, David Holt, Rick Brinkley, Harry Coates, Kim David, Judy Eason McIntyre, Jerry Ellis, John Ford, Jim Halligan, Charlie Laster, and Charles Wyrick.