Biol. Pharm. Bull. 34(7): 1072-1077 (2011)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluating China's Fossil-Fuel CO2 Emissions from A
Evaluating China's fossil-fuel CO2 emissions from a comprehensive dataset of nine inventories Pengfei Han1*, Ning Zeng2*, Tom Oda3, Xiaohui Lin4, Monica Crippa5, Dabo Guan6,7, Greet Janssens-Maenhout5, Xiaolin Ma8, Zhu Liu6,9, Yuli Shan10, Shu Tao11, Haikun Wang8, Rong Wang11,12, 5 Lin Wu4 , Xiao Yun11, Qiang Zhang13, Fang Zhao14, Bo Zheng15 1State Key Laboratory of Numerical Modeling for Atmospheric Sciences and Geophysical Fluid Dynamics, Institute of Atmospheric Physics, Chinese Academy of Sciences, Beijing, China 2Department of Atmospheric and Oceanic Science, and Earth System Science Interdisciplinary Center, University of Maryland, College Park, Maryland, USA 10 3Goddard Earth Sciences Research and Technology, Universities Space Research Association, Columbia, MD, United States 4State Key Laboratory of Atmospheric Boundary Layer Physics and Atmospheric Chemistry, Institute of Atmospheric Physics, Chinese Academy of Sciences, Beijing, China 5European Commission, Joint Research Centre (JRC), Directorate for Energy, Transport and Climate, Air and Climate Unit, Ispra (VA), Italy 15 6Department of Earth System Science, Tsinghua University, Beijing, China 7Water Security Research Centre, School of International Development, University of East Anglia, Norwich, UK 8State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing, China 9Tyndall Centre for Climate Change Research, School of International Development, University of East Anglia, Norwich, 20 UK 10Energy and Sustainability -
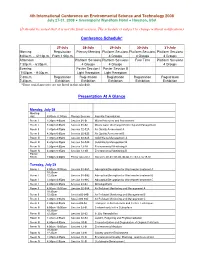
Preliminary Presentation Schedule
4th International Conference on Environmental Science and Technology 2008 July 27-31, 2008 Greenspoint Wyndham Hotel Houston, USA (It should be noted that it is not the final version. The schedule is subject to change without notification) Conference Schedule* 27-July 28-July 29-July 30-July 31-July Morning Registration Plenary Meeting Platform Sessions Platform Sessions Platform Sessions 8:00a.m. - 12:10p.m. From 1:00p.m. 4 Groups 4 Groups 4 Groups Afternoon Platform Sessions Platform Sessions Free Time Platform Sessions 1:30p.m. - 6:05p.m. 4 Groups 4 Groups 4 Groups Evening Poster Session I Poster Session II 7:00p.m. - 9:00p.m. Light Reception Light Reception 7:30a.m. - Registration Registration Registration Registration Registration 7:30p.m. Exhibition Exhibition Exhibition Exhibition Exhibition *Some social activities are not listed in this schedule. Presentation At A Glance Monday, July 28 Meeting Hall 9:00am-11:50am Plenary Session Keynote Presentation Room I 1:30pm-4:05pm Session 01-01 Water Resouces and Assessment Room I 4:30pm-6:05pm Session 01-02 Waste water Discharge Monitoring and Management Room II 1:30pm-4:05pm Session 02-02A Air Quality Assessment A Room II 4:30pm-6:05pm Session 02-02B Air Quality Assessment B Room III 1:30pm-4:05pm Session 03-03A Solid Waste Management A Room III 4:30pm-6:05pm Session 03-03B Solid Waste Management B Room IV 1:30pm-4:05pm Session 13-2A Environmental Monitoring A Room IV 4:30pm-6:05pm Session 13-2B Environmental Monitoring B Poster Room 7:00pm-9:00pm Poster Session I Sessions 01-01~01-05, -

Shanxi Takes Stock of Past Achievements
12 | DISCOVER SHANXI Friday, January 29, 2021 CHINA DAILY Shanxi takes stock of past achievements Provincial congress hails success in poverty alleviation, plans economic transformation By YUAN SHENGGAO To make the transformation pos sible, Shanxi has developed 62 new he past five years, the 13th comprehensive transformation FiveYear Plan (201620), demonstration zones in the past was a crucial period for five years. This compares with just Shanxi province to develop 26 such zones in earlier years. Tan allaround xiaokang, or moder The combined area of the 88 ately prosperous, society, said Lin demonstration zones is 2,881 Wu, governor of Shanxi, at the square kilometers, or 1.85 percent recent session of the provincial peo of Shanxi’s total land area. The ple’s congress. industrial added value of these The fourth session of 13th Shanxi zones took up 35 percent of the pro People’s Congress was held in the vincial total. provincial capital of Taiyuan from Lin said Shanxi regards techno Jan 2023. logical innovation as a major driver The provincial legislature, the for local growth. Five key national Shanxi People’s Congress, with 517 laboratories and 31 corporate tech delegates present at the session, nological centers were established endorsed the work report of the in Shanxi over the past five years. provincial government, the prov The number of certificated high ince’s 14th FiveYear Plan (202125) tech enterprises in 2020 was more and the longrange objectives for than 3.5 times that of 2015. the period ending in 2035, as well as The governor said the province new local regulations. -

Information to Users
INFORMATION TO USERS This manuscript Pas been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissenation copies are in typewriter face, while others may be from anytype of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely. event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material bad to beremoved, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections with smalloverlaps. Each original is also photographed in one exposure and is included in reduced form at the back ofthe book. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. UMI A Bell &Howell Information Company 300North Zeeb Road. Ann Arbor. MI48106-1346 USA 313!761-47oo 800:521·0600 THE LIN BIAO INCIDENT: A STUDY OF EXTRA-INSTITUTIONAL FACTORS IN THE CULTURAL REVOLUTION A DISSERTATION SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAII IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN HISTORY AUGUST 1995 By Qiu Jin Dissertation Committee: Stephen Uhalley, Jr., Chairperson Harry Lamley Sharon Minichiello John Stephan Roger Ames UMI Number: 9604163 OMI Microform 9604163 Copyright 1995, by OMI Company. -

Towards a Psychobiographical Study of Lin Yutang
What Maketh the Man? Towards a Psychobiographical Study of Lin Yutang Roslyn Joy Ricci School of Social Science/Centre for Asian Studies University of Adelaide November 2013 Abstract Dr Lin Yutang, philologist, philosopher, novelist and inventor was America’s most influential native informant on Chinese culture from the mid-1930s to the mid-1950s. Theoretical analysis of Lin’s accomplishments is an ongoing focus of research on both sides of the North Pacific: this study suggests why he made particular choices and reacted in specific ways during his lifetime. Psychobiographical theory forms the framework for this research because it provides a structure for searching within texts to understand why Lin made choices that led to his lasting contribution to transcultural literature. It looks at foundational beliefs established in his childhood and youth, at why significant events in adulthood either reinforced or altered these and why some circumstances initiated new beliefs. Lin’s life is viewed through thematic lenses: foundational factors; scholarship and vocation; the influence of women; peer input; and religion, philosophy and humour. Most of his empirical life journey is already documented: this thesis suggests why he felt compelled to act and write as he did. In doing so, it offers possible scenarios of why Lin’s talents developed and why his life journey evolved in a particular manner, place and time. For example, it shows the way in which basic beliefs—formed during Lin’s childhood and youth and later specific events in adulthood—affected his life’s journey. It analyses how his exposure to the theories of Taoism, Confucianism and Buddhism affected his early childhood basic belief—Christianity—and argues that he accommodated traditional Chinese beliefs within Christianity. -

2016 Research Summary
2016 Research Summary Division of Electrical Engineering Department of Electrical Engineering Graduate Institute of Electrical Engineering Graduate Institute of Photonics and Optoelectronics Graduate Institute of Communication Engineering Graduate Institute of Electronics Engineering Graduate Institute of Biomedical Electronic and Bioinformatics College of Electrical Engineering and Computer Science National Taiwan University Taipei, Taiwan, Republic of China CONTENTS Index of Faculty Members Biography Project Abstracts Facutly Publications (Since 2014) INDEX OF FACULTY MEMBERS Chang, Hung-chun Huang, Chung-Yang Chang, Shi-Chung Huang, Ding-Wei Chang, Yao-Wen Huang, JianJang Chen, Ching-Jan Huang, Jiun-Lang Chen, Chung-Ping Huang, Nien-Tsu Chen, Ho-Lin Huang, Polly Chen, Homer H. Huang, Sheng-Lung Chen, Hsin-Shu Huang, Tian-Wei Chen, Jyh-Horng Hwu, Jenn-Gwo Chen, Liang-Gee Jeng, Shyh-Kang Chen, Ming-Syan Jiang, Jie-Hong R. Chen, Sao-Jie Kiang, Jean-Fu Chen, Shih-Yuan Kiang, Yean-Woei Chen, Yaow-Ming Kuan, Chieh-Hsiung Chen, Yi-Jan Emery Kuo, James B. Chen, Yung-Yaw Kuo, Po-Ling Cheng, Chen-Mou Kuo, Sy-Yen Cheng, I-Chun Lai, Feipei Chien, Shao-Yi Lee, Hsinyu Chiou, Yih-Peng Lee, Hung-Yi Chiueh, Tzi-Dar Lee, Jiun-Haw Choi, Wing-Kit Lee, Jri Chou, Chun-Ting Lee, Ju-Hong Chou, Hsi-Tseng Lee, Lin-shan Chu, Tah-Hsiung Lee, Si-Chen Chuang, Eric Y. Lee, Tai-Cheng Chung, Char-Dir Lei, Chin-Laung Chung, Hsiao-Wen Li, Chien-Mo Ding, Jian-Jiun Li, Jiun-Yun Fu, Li-Chen Li, Pai-Chi Hsieh, Hung-Yun Lian, Feng-Li Hsu, Yuan-Yih Liao, Wanjiun Lin, Chih-Ting Sun, Chi-Kuang Lin, Chii-Wann Sung, Kung-Bin Lin, Ching-Fuh Tian, Wei-Cheng Lin, Gong-Ru Tsai, Jui-che Lin, Hao-Hsiung Tsai, Kuen-Yu Lin, Hoang Yan Tsai, Zsehong Lin, Kun-You Tsao, Hen-Wai Lin, Mao-Chao Tseng, Snow H. -

Latest CPC Measures Publicized in Shanxi
6 | DISCOVER SHANXI Thursday, December 3, 2020 HONG KONG EDITION | CHINA DAILY Latest CPC measures publicized in Shanxi New industrial growth pattern, rural development, environmental protection and deepening reforms high on provincial authorities’ agenda own innovative plans based on the spirit of the plenary session. “In the next five years and beyond, poverty reduction, balanced eco- nomic development and environ- mental protection will be the priorities of the government’s agen- da,” Yang said. Yang Xiaoshan, Party secretary of By YUAN SHENGGAO Xin’anquan township in the city, said she is happy with the agricul- The Fifth Plenary Session of the tural and rural development-related 19th Central Committee of the Com- policies proposed by the plenary munist Party of China was held in session. Beijing from Oct 26 to 29. “As an official working for agricul- The session adopted the CPC Cen- ture and rural affairs, I’m fully tral Committee’s proposals for the aware of the importance of my mis- formulation of the 14th Five-Year sion,” Yang Xiaoshan said. “We have Plan (2021-25) for National Eco- made great achievements in poverty nomic and Social Development and alleviation this year and we will the Long-Range Objectives Through devote more efforts to creating a the Year 2035, according to a com- well-off society in our town.” munique released from the confer- Feng Zhijun, chief of the Shanxi ence. State-owned Assets Supervision and By outlining the blueprints for Administration Commission, pre- national economic and social devel- sided over a meeting on Nov 18 in opment for the next five years and Shanxi Coking Coal Group based in long-term goals for the next 15 years, Taiyuan, the provincial capital of the plenary session provided an Shanxi. -

Wu Tiecheng in the 1911 Revolution in Jiujiang, China
KEMANUSIAAN Vol. 23, No. 1, (2016), 65–96 Guanxi, Networks and Eloquence: Wu Tiecheng in the 1911 Revolution in Jiujiang, China TAN CHEE SENG Department of Chinese Studies, Faculty of Arts and Social Sciences, National University of Singapore, The Shaw Foundation Building, Block AS7, Level 3, 5 Arts Link, Singapore 117570 [email protected] Abstract. This paper aims to examine how Wu Tiecheng (1888–1953), one of the Kuomintang (KMT) and Republican China senior statesmen, had established and developed his three crucial assets: guanxi ( 关系 relationships or connections), networks and eloquence since his formative years (1888–1910) until the 1911 Revolution in Jiujiang, China. His uniqueness as a revolutionary who was equipped with those three elements nurtured during the formative years of his youth was subsequently reflected during the revolution. Concurrently, this will explore prior and during the revolution, what types of guanxi and networks he had tried to establish, subsequently develop and even utilise. Furthermore, this paper also attempts to look into how those guanxi, networks and even eloquence had assisted Wu in his endeavours to become one of the leading revolutionaries in this revolution. In probing into the above mentioned aspects, it would trace the activities he had involved and roles played. Three essential elements apart from moulding and influencing Wu, had become part of his social capital which benefitted him and continuously to be utilised in the coming years. Those had given impetus to his rise within the KMT and Republican political circles, and also made him a distinct figure among peers. Keywords and phrases: Wu Tiecheng, 1911 Revolution, guanxi, networks, eloquence © Penerbit Universiti Sains Malaysia, 2016 66 Tan Chee Seng Introduction Figure 1. -

Evaluating China's Fossil-Fuel CO2 Emissions from a Comprehensive
Atmos. Chem. Phys., 20, 11371–11385, 2020 https://doi.org/10.5194/acp-20-11371-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Evaluating China’s fossil-fuel CO2 emissions from a comprehensive dataset of nine inventories Pengfei Han1, Ning Zeng2, Tom Oda3, Xiaohui Lin4, Monica Crippa5, Dabo Guan6,7, Greet Janssens-Maenhout5, Xiaolin Ma8, Zhu Liu6,9, Yuli Shan10, Shu Tao11, Haikun Wang8, Rong Wang11,12, Lin Wu4, Xiao Yun11, Qiang Zhang13, Fang Zhao14, and Bo Zheng15 1State Key Laboratory of Numerical Modeling for Atmospheric Sciences and Geophysical Fluid Dynamics, Institute of Atmospheric Physics, Chinese Academy of Sciences, Beijing, China 2Department of Atmospheric and Oceanic Science, and Earth System Science Interdisciplinary Center, University of Maryland, College Park, MD, USA 3Goddard Earth Sciences Research and Technology, Universities Space Research Association, Columbia, MD, USA 4State Key Laboratory of Atmospheric Boundary Layer Physics and Atmospheric Chemistry, Institute of Atmospheric Physics, Chinese Academy of Sciences, Beijing, China 5European Commission, Joint Research Centre (JRC), Directorate for Energy, Transport and Climate, Air and Climate Unit, Ispra (VA), Italy 6Department of Earth System Science, Tsinghua University, Beijing, China 7Water Security Research Centre, School of International Development, University of East Anglia, Norwich, UK 8State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing, China -

1912-06 Personnel
WEEKLY PERSONNEL UPDATE 6 December 2019 • Massive transfers across regions The week of 2 December has witnessed a series of provincial transfers, including: Of all the appointments above, Lin Keqing’s appointment is to be highlighted. The latest transfer to Guangdong, although on the same level and in the same job, has made Lin the youngest CCPSC member of Guangdong. More importantly, Lin was a Beijing official throughout his career before the latest appointment—his movement from the capital of China to the most economically-advanced province indicates favor of top leadership as this is a clear attempt to nurture Lin for higher offices. At the same time, He Zhongyou has become the second Guangdong provincial leader transferred to Hainan in recent years. Former Guangdong Vice Governor Lan Fo’an (蓝佛安) was promoted to Hainan in March 2017 as CCPSC member and Commission for Discipline Inspection Chairman. With He’s latest transfer to Hainan, the two large cities of the island- province, Haikou and Sanya, are now both led by reformists from economically better-off regions. Incumbent Sanya Party Secretary is Tong Daochi (童道驰), a banker-turned official with a doctoral degree from Pardee RAND Graduate School and over six years of experience in the World Bank following his graduation. These transfers, alongside the prior transfer of hundreds of officials to Hainan, indicate again that Beijing wants to expedite the reform of Hainan that could turn the island-province into an international tourism hub. At the same time, the political jockeying surrounding the top provincial jobs had also begun (some of the appointments below were already covered in the earlier briefings). -

Chinese Politics and Society Now Available on Worldscinet
Chinese Politics and Society Now available on WorldSciNet The Construction of Democracy China’s Theory, Strategy and Agenda by Shangli Lin (Fudan University, China) The book expounds on the role played by democracy in China’s revolution and modernization led by the Communist Party of China (CPC), and how the CPC, in both its party building and state building, has constantly sought to leverage democracy’s positive functions while avoiding its shortcomings. Special attention is paid to reconstructing and explaining the historical contexts from which the Party’s theoretical innovations have emerged, thus offering readers insights into the inner political logic that has shaped China’s development. The author, a member of the Party’s senior policy panel, offers a perceptive analysis of the modernization of the country and its governing capacity, and Political Development in Hong Kong provides a clear assessment of how democracy in China has developed with by Joseph Yu-shek Cheng the times. This volume analyzes the political Always bearing the big picture in mind, the author has not shied away from development in Hong Kong in some of the more controversial parts of China’s recent history, and his deep chronological order from the Sino- understanding of relevant Party documents and historical facts give strong British negotiations till today. It support to his analyses. He concludes that that the Party is central to leading focuses on the rule of the British the nation to explore its path of socialism with Chinese characteristics and that administration before 1997; the the country has always emerged stronger after setbacks. -

Heroes' Welcome for Hundreds of Medics Back Home in Shanxi
20 | DISCOVER SHANXI Friday, March 27, 2020 CHINA DAILY Philanthropists sending supplies over the world By YUAN SHENGGAO said an official of the Shanxi Feder ation of Returned Overseas Chi When the novel coronavirus nese. pneumonia recently escalated into The supplies overseas are not a pandemic, businesses and indi limited to Chinese expats. viduals in North China’s Shanxi Through overseas branches of province once again offered to the Shanxi Construction Invest help. ment Group, the Shanxi Red On March 30, a batch of medical Cross Society has delivered 3,500 supplies from Shanxi set off over sets of protective clothing to seas. The supplies — including Japan, South Korea, Italy and 30,000 N95 face masks and 13,200 Germany. packs of traditional Chinese medi In addition, the society donated cines — were sent to Chinese stu 6.32 million yuan ($893,600) to a dents and expatriates in more than Chinese medical rescue team in 30 countries such as Italy, the Unit Italy so it could buy intensive care ed Kingdom, Canada, the United unit equipment for treating local States, Brazil, Japan, Singapore patients. and Thailand. “There is no boundary for dis The delivery was organized by ease control, as all the countries the Shanxi Red Cross Society and in the world are a community the Shanxi Federation of Returned with a shared future,” said an Overseas Chinese, after they official with the Shanxi Red received donations from local Cross Society. companies and residents. In recent days, staff members of Zhang Yiqin, a Shanxi native the provincial Red Cross society who lives in Houston, the US, was and the federation of returned ready to receive the supplies and overseas Chinese are receiving distribute them to local Chinese more donations from local busi A total of 662 medics have arrived in Shanxi by March 23 after completing their rescue missions in Hubei province.