Molecular Phylogenetics of the Rhinoceros Clade And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Importance of Wallows to Javan Rhino Ecology and Behaviour
RESEARCH More than just mud: the importance of wallows to Javan rhino ecology and behaviour Steven G Wilson1,2*,Georgina Hockings1, Jo-Anne M Deretic2, Salit Kark1 1The Biodiversity Research Group, The School of Biological Sciences, Centre for Biodiversity and Conservation Science, The University of Queensland, Brisbane, QLD, 4072 Australia 2Land, Biodiversity and Indigenous and River Health Programs, Goulburn Broken Catchment Management Authority, 168 Welsford Street, Shepparton, VIC 3632 Australia *corresponding author: [email protected] Abstract All members of the family Rhinocerotidae have the need to wallow in mud or water to protect their skin from sun damage, remove ectoparasites and for thermoregulation purposes. Just 72 wild Javan rhino (Rhinoceros sondaicus) remain on the planet, all located in their last stronghold in Ujung Kulon National Park (UKNP), West Java, Indonesia. Javan rhinos need to wallow regularly throughout the year, yet the role wallows play in their behaviour and the importance to the species remains little understood. In this study, we identified, mapped and studied 35 wallows in eastern UKNP, where rhinos were active. We spatially mapped and recorded each wallow’s characteristics. We examined rhino wallowing behaviour using 392 remote camera trap videos, taken across UKNP during a five-year study from 2011 to 2016. We identified and categorised eight behavioural patterns at and near wallows related to rhino daily activities and found that wallows have several key features for the Javan rhinos. Findings revealed that Javan rhinos, who construct the wallows themselves, choose sites with 75% shade cover and often at an elevation. Analysis of the rhino calls from camera trap videos taken at and near wallows, identify seven vocalisation descriptors with accompanying sonograms, a first for this rare and shy rainforest species. -

SMC 136 Gazin 1958 1 1-112.Pdf
SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 136, NUMBER 1 Cftarlesi 3B, anb JKarp "^aux OTalcott 3^es(earcf) Jf unb A REVIEW OF THE MIDDLE AND UPPER EOCENE PRIMATES OF NORTH AMERICA (With 14 Plates) By C. LEWIS GAZIN Curator, Division of Vertebrate Paleontology United States National Museum Smithsonian Institution (Publication 4340) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION JULY 7, 1958 THE LORD BALTIMORE PRESS, INC. BALTIMORE, MD., U. S. A. CONTENTS Page Introduction i Acknowledgments 2 History of investigation 4 Geographic and geologic occurrence 14 Environment I7 Revision of certain lower Eocene primates and description of three new upper Wasatchian genera 24 Classification of middle and upper Eocene forms 30 Systematic revision of middle and upper Eocene primates 31 Notharctidae 31 Comparison of the skulls of Notharctus and Smilodectcs z:^ Omomyidae 47 Anaptomorphidae 7Z Apatemyidae 86 Summary of relationships of North American fossil primates 91 Discussion of platyrrhine relationships 98 References 100 Explanation of plates 108 ILLUSTRATIONS Plates (All plates follow page 112) 1. Notharctus and Smilodectes from the Bridger middle Eocene. 2. Notharctus and Smilodectes from the Bridger middle Eocene. 3. Notharctus and Smilodectcs from the Bridger middle Eocene. 4. Notharctus and Hemiacodon from the Bridger middle Eocene. 5. Notharctus and Smilodectcs from the Bridger middle Eocene. 6. Omomys from the middle and lower Eocene. 7. Omomys from the middle and lower Eocene. 8. Hemiacodon from the Bridger middle Eocene. 9. Washakius from the Bridger middle Eocene. 10. Anaptomorphus and Uintanius from the Bridger middle Eocene. 11. Trogolemur, Uintasorex, and Apatcmys from the Bridger middle Eocene. 12. Apatemys from the Bridger middle Eocene. -

The Interspecific Relationships of Black Rhinoceros (Diceros Bicornis) in Hluhluwe-Imfolozi Park
The interspecific relationships of black rhinoceros (Diceros bicornis) in Hluhluwe-iMfolozi Park Roan David Plotz B.Sc. (ConsBiolEcol) (Hons1); GradDipEd (Sec) A thesis submitted to Victoria University of Wellington in fulfilment of the requirement for the degree of Doctor of Philosophy in Ecology and Biodiversity 2014 1 “To Ryker, may the wild places of this world long remain protected to captivate and inspire you” Black rhino near the Black iMfolozi River in Hluhluwe-iMfolozi Park, Zululand, South Africa (Photograph by Dale Morris). “We learn more by looking for the answer to a question and not finding it than we do from learning the answer itself.” Lloyd Alexander 2 ABSTRACT As habitat loss, predators (human and non-human) and disease epidemics threaten species worldwide, protected sanctuaries have become vital to species conservation. Hluhluwe-iMfolozi Park (HiP) in South Africa is at the centre of one of the world’s greatest conservation success stories. The formal proclamation of HiP in 1895 prevented the extinction of the south-central black rhino (Diceros bicornis minor) population. In recent times HiP has been a strategic source population for the D. b. minor range expansion program, facilitating an 18-fold population increase across southern Africa. However, HiP’s own black rhino population appears to be in decline. Evidence for decline is most often attributed to overpopulation and poor habitat quality that is driving apparently significant increases in the average home range sizes, poor growth rates (i.e., low calf recruitment) and poor body condition of black rhino. Other factors such as non-human calf predation and parasitism have also been raised as potential causes of decline but remain untested. -

Bill Analysis for File Copy
OLR Bill Analysis sSB 925 AN ACT PROHIBITING THE IMPORT, SALE AND POSSESSION OF AFRICAN ELEPHANTS, LIONS, LEOPARDS, BLACK RHINOCEROS, WHITE RHINOCEROS AND GIRAFFES. SUMMARY This bill generally bans importing, possessing, selling, offering for sale, or transporting in Connecticut a specimen (dead or alive) of any of six types of African animals, which the bill collectively refers to as the “big six African species.” It applies to certain elephants, lions, leopards, giraffes, and two rhinoceros species. The bill establishes a graduated penalty structure for violations, ranging from no penalty for someone who, unaware and in good faith, violates the ban, to a class D felony for someone with at least two prior violations subject to penalty. In all cases, the bill requires seizing the specimen and any other property or item used in connection with the violation. The specimen, property, or item is then forfeited and, unless the specimen is alive, destroyed. The bill contains several exemptions, including for a specimen that is already legally in the state or distributed to a beneficiary or heir, as long as the owner or distributee timely obtains a certificate of possession from the Department of Energy and Environmental Protection (DEEP). The ban also does not apply to fossils and ivory and the following under certain conditions: circuses; museums; zoological institutions; and motion picture, television, or digital media production companies. Lastly, the bill specifies that the ban does not prohibit transporting through the state endangered or threatened species subject to the terms of another state’s permit, which existing law allows. The United States regulates the trade of the species covered by the Researcher: KLM Page 1 5/8/21 2021SB-00925-R010637-BA.DOCX bill, except the African giraffe, through the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) and laws such as the Endangered Species Act (16 U.S.C. -
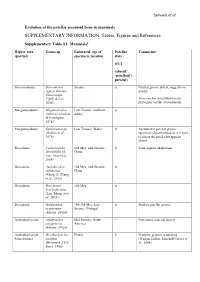
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp. -

Early Eocene Fossils Suggest That the Mammalian Order Perissodactyla Originated in India
ARTICLE Received 7 Jul 2014 | Accepted 15 Oct 2014 | Published 20 Nov 2014 DOI: 10.1038/ncomms6570 Early Eocene fossils suggest that the mammalian order Perissodactyla originated in India Kenneth D. Rose1, Luke T. Holbrook2, Rajendra S. Rana3, Kishor Kumar4, Katrina E. Jones1, Heather E. Ahrens1, Pieter Missiaen5, Ashok Sahni6 & Thierry Smith7 Cambaytheres (Cambaytherium, Nakusia and Kalitherium) are recently discovered early Eocene placental mammals from the Indo–Pakistan region. They have been assigned to either Perissodactyla (the clade including horses, tapirs and rhinos, which is a member of the superorder Laurasiatheria) or Anthracobunidae, an obscure family that has been variously considered artiodactyls or perissodactyls, but most recently placed at the base of Proboscidea or of Tethytheria (Proboscidea þ Sirenia, superorder Afrotheria). Here we report new dental, cranial and postcranial fossils of Cambaytherium, from the Cambay Shale Formation, Gujarat, India (B54.5 Myr). These fossils demonstrate that cambaytheres occupy a pivotal position as the sister taxon of Perissodactyla, thereby providing insight on the phylogenetic and biogeographic origin of Perissodactyla. The presence of the sister group of perissodactyls in western India near or before the time of collision suggests that Perissodactyla may have originated on the Indian Plate during its final drift toward Asia. 1 Center for Functional Anatomy & Evolution, Johns Hopkins University School of Medicine, 1830 E. Monument Street, Baltimore, Maryland 21205, USA. 2 Department of Biological Sciences, Rowan University, Glassboro, New Jersey 08028, USA. 3 Department of Geology, H.N.B. Garhwal University, Srinagar 246175, Uttarakhand, India. 4 Wadia Institute of Himalayan Geology, Dehradun 248001, Uttarakhand, India. 5 Research Unit Palaeontology, Ghent University, Krijgslaan 281-S8, B-9000 Ghent, Belgium. -

Listing the Southern White Rhino
Federal Register / Vol. 78, No. 176 / Wednesday, September 11, 2013 / Rules and Regulations 55649 that the device must be registered; may as part of the equipment certification booster operators have the proper only be operated with the consent of the process. The R&O also requires that if a authority to operate their devices. consumer’s wireless provider; may only manufacturer claims that a device will Federal Communications Commission. be operated with approved antennas not affect E911 communications, the Marlene H. Dortch, and cables; and that E911 manufacturer must certify this claim communications may be affected for during the equipment certification Secretary. calls served by using the device. process. Note: The ‘‘application for [FR Doc. 2013–22121 Filed 9–10–13; 8:45 am] Industrial Signal Boosters must include equipment’’ certification requirements BILLING CODE 6712–01–P a label stating that the device is not a are met under OMB Control Number consumer device, is designed for 3060–0057, FCC Form 731. installation by FCC licensees or a DEPARTMENT OF THE INTERIOR Antenna Kitting Documentation qualified installer, and the operator Requirement must have a FCC license or consent of Fish and Wildlife Service a FCC licensee to operate the device. Sections 20.21(e)(8)(i)(G), Accordingly, all signal boosters 20.21(e)(9)(i)(H)—The rules require that 50 CFR Part 17 marketed on or after March 1, 2014, all consumer boosters must be sold with user manuals specifying all antennas [Docket Number FWS–HQ–ES–2013–0055; must include the advisories (1) In on- FXES111809F2070B6] line point-of-sale marketing materials; and cables that meet the requirements of (2) in any print or on-line owner’s this section. -

The Interspecific Relationships of Black Rhinoceros (Diceros Bicornis) in Hluhluwe-Imfolozi Park
The interspecific relationships of black rhinoceros (Diceros bicornis) in Hluhluwe-iMfolozi Park Roan David Plotz B.Sc. (ConsBiolEcol) (Hons1); GradDipEd (Sec) A thesis submitted to Victoria University of Wellington in fulfilment of the requirement for the degree of Doctor of Philosophy in Ecology and Biodiversity 2014 1 2 “To Ryker, may the wild places of this world long remain protected to captivate and inspire you” Black rhino near the Black iMfolozi River in Hluhluwe-iMfolozi Park, Zululand, South Africa (Photograph by Dale Morris). “We learn more by looking for the answer to a question and not finding it than we do from learning the answer itself.” Lloyd Alexander 3 4 ABSTRACT As habitat loss, predators (human and non-human) and disease epidemics threaten species worldwide, protected sanctuaries have become vital to species conservation. Hluhluwe-iMfolozi Park (HiP) in South Africa is at the centre of one of the world’s greatest conservation success stories. The formal proclamation of HiP in 1895 prevented the extinction of the south-central black rhino (Diceros bicornis minor) population. In recent times HiP has been a strategic source population for the D. b. minor range expansion program, facilitating an 18-fold population increase across southern Africa. However, HiP’s own black rhino population appears to be in decline. Evidence for decline is most often attributed to overpopulation and poor habitat quality that is driving apparently significant increases in the average home range sizes, poor growth rates (i.e., low calf recruitment) and poor body condition of black rhino. Other factors such as non-human calf predation and parasitism have also been raised as potential causes of decline but remain untested. -

The Decline of Elephant and Black Rhinoceros in Ethiopia M
The decline of elephant and black rhinoceros in Ethiopia M. J. Largen and D. W. Yalden From the authors' review of the mammals of Ethiopia, undertaken during the last 14 years, it is clear that Ethiopia's elephants and rhinoceroses have suffered massive declines since the beginning of the century. There are perhaps 9000 elephants left, but poaching for ivory and agricultural encroachment continue to threaten them, and only a few rhinoceroses still survive. Ethiopia has limited resources to protect these remaining animals, and needs help and encouragement from outside its boundaries. The status of the elephant Loxodonta africana borders of the country. Destruction of natural and that of the black rhinoceros Diceros bicomis habitat and harassment by an ever-expanding in Africa continue to give acute concern to con- human population with an ever-increasing servationists. Concern for the elephant popu- demand for land have undoubtedly contributed lation includes worries about declining range, to this situation, but enormous numbers of and whether the ivory trade is taking an exploit- elephants have also been killed for profit. The able surplus or is itself causing populations to ivory trade was already long established in 1899 decline (Parker and Martin, 1982, 1983). In a when Harrison (1901) reported 1500 tusks being recent report, Western and Vigne (1985) sug- sent to the capital, in the two months preceding gested that the total black rhinoceros population his visit, from the southern region of the of the continent was only 8800 in 1984, a drop of Ethiopian Rift Valley where the species has since 40 per cent from the 14,000-15,000 of 1980. -

A New Genus of Rhinocerotoid from The
386 JOURNAL OF VERTEBRATEPALEONTOLOGY, VOL. 17, NO. 2, 1997 B C D FIGURE 2. Skulls of Uintaceras. UCMP 69722 in A, dorsal and B, left lateral views. UW 2410 in C, left lateral and D, dorsal views. Scale bar = 5 cm. damaged, and little can be said of their morphology beyond size. All four premolars are present on both sides; no molari- that they appear not to be molariform. The upper premolars are zation is evident, and they are similar to those of CM 2908 and better preserved on UCMP 69722 and UW 2410 (Fig. 3G and UCMP 69722 (Fig. 5B, C). Radinsky (1967; see fig. 11) noted 3I), and these specimens show more clearly the nonmolariform the presence of an isolated entoconid on p3 and p4 in UCMP condition of the upper premolars. 69722, a characteristic absent in species of Forstercooperia. On The upper molars (Fig. 3F) have come free of the maxillae, CM 12004, p3 and p4 are too worn to determine if the ento- but all six are present. Ml is the most worn on both sides, and conid is present and isolated. The lower molars of CM 12004 the left Ml is damaged. They are generally similar to those of (Fig. 5A) are essentially indistinguishable from those of CM Hyrachyus, the main differences being the smaller size, pres- 2908, UCMP 69722, and UCMP 69370. ence of a lingual cingulum, and relatively large parastyles of Vertebrae-An indeterminate number of vertebrae are pres- Hyrachyus. Much as in Hyrachyus, the metacone on M3 of CM ent in CM 12004, but all are either heavily distorted and dam- 12004 is larger and placed more labially than in Hyracodon; it aged, or are still embedded in matrix. -

Rhinoceros & Tiger Conservation
U.S. Fish & Wildlife Service Rhinoceros & Tiger Conservation Act Summary Report 2001-2003 1 The U.S. Fish and Wildlife Service’s mission is working with others to conserve, protect and enhance fish, wildlife, and plants and their habitats for the continuing benefit of the American people. We are the only agency of the U.S. Government with that primary mission. The Service also supports the Department of the Interior’s Strategic Plan to involve various partners such as State and local governments, communities, federally recognized Tribes, non-governmental organizations, and private citizens. The Service’s Division of International Conservation and its partners worldwide support these goals through cross-border cooperation to preserve the habitats that sustain migratory and endangered species. The leadership, knowledge, and cooperation of international partners is crucial to ensure the global conservation of these species and their habitats. Front: Greater one-horned rhinoceros in tall grass habitat of Kaziranga National Park in India’s northeastern state of Assam. International Rhino Foundation Rhinoceros & Tiger Conservation Act Summary Report 2001-2003 A child living in the vicinity of Russia’s tiger habitat created this painting for an art competition. The competition was a component of an education program supported by the Rhinoceros and Tiger Conservation Fund to develop support for tiger conservation among young people. Artwork property of Khabarovsk Wildlife Foundation Government and non-government personnel of Lao PDR, working with Wildlife Conservation Society staff (top), are conducting a tiger camera trap survey (center) of the country’s most promising tiger habitat. Resulting camera trap photographs include the tiger (bottom) and its prey, a sambhar (right), in Nam Et Phou Louey National Protected Area. -

1999-2000 Summary Report
U.S. Fish & Wildlife Service Rhinoceros & Tiger Conservation Act Summary Report 1999-2000 “The mission of the U.S. Fish and Wildlife Service is working with others to conserve, protect and enhance fish, wildlife, plants and their habitats for the continuing benefit of the American people.” Cover: Black rhino © Corel Professional Photo Rhinoceros & Tiger Conservation Act Summary Report 1999-2000 Above: Page from storybook on Vietnamese rhino produced with support from the Rhinoceros and Tiger Conservation Fund. See page 17. ©Ina Becker and Trung Dung, Cat Tien National Park Conservation Project Introduction “The tiger is Rhinos and tigers are grand beasts! Their charisma included them in the heritage of more than a many cultures. They have made their way into storybooks, religions, medicines, and charismatic ad campaigns. In their native habitats they predator: it represent beauty, power, grace, and a world kept in balance by the forces of is a keystone nature rather than the whims of man. species in its However, our attraction to these species environment. and their habitats also threatens their existence. It has led to their killing for By saving the trophies and medicines and to the fragmentation and outright destruction of tiger in the their habitat by people seeking timber and world, we save land resources. They are now among the world’s most endangered species. complex ecosystems and habitats that would other- wise be destroyed in the relentless march of human need and, all too often, greed.” Richard Burge Riding theTiger* *Reprinted with the permission of Cambridge University Press Left: Large blocks of the Amur tiger’s forest habitat remain in northern China adjacent to Russian tiger habitat.