Incorporating COVID-19 Related Organ Failure in Candidate Listings OPTN Lung Transplantation Committee
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lung Transplantation with the OCS (Organ Care System)
Lung Transplantation with the OCSTM (Organ Care System) Lung System Bringing Breathing Lung Preservation to Transplant Patients A Guide for You and Your Family DRAFT ABOUT THIS BOOKLET This booklet was created for patients like you who have been diagnosed with end-stage lung failure and are candidates for a lung transplant. It contains information that will help you and your family learn about options available to you for a transplant. This booklet includes information on your lungs, how they function, and respiratory failure. In addition, you will learn about a new way to preserve lungs before transplantation, called breathing lung preservation. Your doctor is the best person to explain your treatment options and their risks and to help you decide which option is right for you. The booklet explains: • Breathing lung preservation with the OCS™ Lung System • How the OCS™ Lung System works • Who is eligible for the OCS™ Lung System • Lung transplant complications • How the lungs function • What is respiratory failure and the treatment options • What to expect during your treatment • Summary of clinical data for the OCS™ Lung System • Contact Information Please read this booklet carefully and share it with your family and caregivers. For your convenience, a glossary is provided in the front of this booklet. Terms in the text in bold italics are explained in the glossary. If you have questions about the OCS™ Lung System that are not answered in this booklet, please ask your physician. This booklet is intended for general information only. It is not intended to tell you everything you need to know about a lung transplant. -

Case Report AJNT
Arab Journal of Nephrology and Transplantation. 2011 Sep;4(3):155-8 Case Report AJNT High Ureteric Injury Following Multiorgan Recovery: Successful Kidney Transplant with Boari Flap Ureterocystostomy Reconstruction Michael Charlesworth*, Gabriele Marangoni, Niaz Ahmad Department of Transplantation, Division of Surgery, St James’s University Hospital, Leeds, United Kingdom Abstract Keywords: Kidney; Transplant; Ureter; Donor efficiency Introduction: Despite increased utilization of marginal organs, there is still a marked disparity between organ The authors declared no conflict of interest supply and demand for transplantation. To maximize resources, it is imperative that procured organs are in Introduction good condition. Surgical damage at organ recovery can happen and organs are sometimes discarded as a result. Despite the extension of the donor pool with the inclusion We describe a damaged recovered kidney with high of marginal organs and the use of organs donated after ureteric transection that was successfully transplanted cardiac death, there is still a great disparity between the using a primary Boari flap ureterocystostomy. number of patients on the transplant waiting list and the number of kidney transplants performed each year. Case report: The donor kidney was procured form a It is therefore of paramount importance to maximize deceased donor and sustained damage by transection our scarce resources and avoid the discard of otherwise of the ureter just distal to the pelvi-ureteric junction at functional kidneys due to iatrogenic injuries at the time organ recovery. The recipient had been on the transplant of multi-organ recovery. Essentially, three types of organ waiting list for eight years and not accepting this kidney damage can potentially occur: vascular, parenchymal would have seriously jeopardized her chance of future and ureteric. -

Pelvic Anatomyanatomy
PelvicPelvic AnatomyAnatomy RobertRobert E.E. Gutman,Gutman, MDMD ObjectivesObjectives UnderstandUnderstand pelvicpelvic anatomyanatomy Organs and structures of the female pelvis Vascular Supply Neurologic supply Pelvic and retroperitoneal contents and spaces Bony structures Connective tissue (fascia, ligaments) Pelvic floor and abdominal musculature DescribeDescribe functionalfunctional anatomyanatomy andand relevantrelevant pathophysiologypathophysiology Pelvic support Urinary continence Fecal continence AbdominalAbdominal WallWall RectusRectus FasciaFascia LayersLayers WhatWhat areare thethe layerslayers ofof thethe rectusrectus fasciafascia AboveAbove thethe arcuatearcuate line?line? BelowBelow thethe arcuatearcuate line?line? MedianMedial umbilicalumbilical fold Lateralligaments umbilical & folds folds BonyBony AnatomyAnatomy andand LigamentsLigaments BonyBony PelvisPelvis TheThe bonybony pelvispelvis isis comprisedcomprised ofof 22 innominateinnominate bones,bones, thethe sacrum,sacrum, andand thethe coccyx.coccyx. WhatWhat 33 piecespieces fusefuse toto makemake thethe InnominateInnominate bone?bone? PubisPubis IschiumIschium IliumIlium ClinicalClinical PelvimetryPelvimetry WhichWhich measurementsmeasurements thatthat cancan bebe mademade onon exam?exam? InletInlet DiagonalDiagonal ConjugateConjugate MidplaneMidplane InterspinousInterspinous diameterdiameter OutletOutlet TransverseTransverse diameterdiameter ((intertuberousintertuberous)) andand APAP diameterdiameter ((symphysissymphysis toto coccyx)coccyx) -

Skin Is Not the Largest Organ
View metadata, citation and similar papers at core.ac.uk brought to you by CORE providedRD by Elsevier Sontheimer - Publisher Connector Skin is Not the Largest Organ 3 content. CHS, JNB, and MAS were involved in Paris, France; Division of Genetics and susceptibility to psoriasis vulgaris. J Invest study supervision. Molecular Medicine, St John’s Institute of Dermatol 134:271–3 Dermatology, Guy’s Hospital, London, UK; Martin MA, Klein TE, Dong BJ et al. (2012) Clinical 1,8 4 Alexander A. Navarini , Guy’s and St Thomas’ NHS Foundation pharmacogenetics implementation consortium Trust, Skin Therapy Research Unit, St John’s Laurence Valeyrie-Allanore2,8, guidelines for HLA-B genotype and abacavir 1 Institute of Dermatology, St Thomas’ Hospital, dosing. Clin Pharmacol Ther 91:734–8 Niovi Setta-Kaffetzi , London, UK; 5King’s College Hospital, London, Jonathan N. Barker1,3,4, UK; 6University Medical Center Freiburg, Navarini AA, Valeyrie-Allanore L, Setta-Kaffetzi N et al. (2013) Rare variations in IL36RN in 1 5 Institute of Medical Biometry and Medical Francesca Capon , Daniel Creamer , severe adverse drug reactions manifesting as 2 6 Informatics, Freiburg, Germany and Jean-Claude Roujeau , Peggy Sekula , 7 acute generalized exanthematous pustulosis. 1 Department of Dermatology, J Invest Dermatol 133:1904–7 Michael A. Simpson , Dokumentationszentrum Schwerer 1 Richard C. Trembath , Hautreaktionen (dZh), Universita¨ts-Hautklinik, Setta-Kaffetzi N, Navarini AA, Patel VM et al. (2013) Maja Mockenhaupt7,8 and Freiburg, Germany Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated 1,3,4,8 8 Catherine H. Smith These authors contributed equally to this work. -

GLOSSARY of MEDICAL and ANATOMICAL TERMS
GLOSSARY of MEDICAL and ANATOMICAL TERMS Abbreviations: • A. Arabic • abb. = abbreviation • c. circa = about • F. French • adj. adjective • G. Greek • Ge. German • cf. compare • L. Latin • dim. = diminutive • OF. Old French • ( ) plural form in brackets A-band abb. of anisotropic band G. anisos = unequal + tropos = turning; meaning having not equal properties in every direction; transverse bands in living skeletal muscle which rotate the plane of polarised light, cf. I-band. Abbé, Ernst. 1840-1905. German physicist; mathematical analysis of optics as a basis for constructing better microscopes; devised oil immersion lens; Abbé condenser. absorption L. absorbere = to suck up. acervulus L. = sand, gritty; brain sand (cf. psammoma body). acetylcholine an ester of choline found in many tissue, synapses & neuromuscular junctions, where it is a neural transmitter. acetylcholinesterase enzyme at motor end-plate responsible for rapid destruction of acetylcholine, a neurotransmitter. acidophilic adj. L. acidus = sour + G. philein = to love; affinity for an acidic dye, such as eosin staining cytoplasmic proteins. acinus (-i) L. = a juicy berry, a grape; applied to small, rounded terminal secretory units of compound exocrine glands that have a small lumen (adj. acinar). acrosome G. akron = extremity + soma = body; head of spermatozoon. actin polymer protein filament found in the intracellular cytoskeleton, particularly in the thin (I-) bands of striated muscle. adenohypophysis G. ade = an acorn + hypophyses = an undergrowth; anterior lobe of hypophysis (cf. pituitary). adenoid G. " + -oeides = in form of; in the form of a gland, glandular; the pharyngeal tonsil. adipocyte L. adeps = fat (of an animal) + G. kytos = a container; cells responsible for storage and metabolism of lipids, found in white fat and brown fat. -

Learning Objectives
Restrictive Lung Diseases & Pulmonary Vascular Disease Pulmonary 2018 RESTRICTIVE LUNG DISEASES AND PULMONARY VASCULAR DISEASE Joel Thibodeaux, MD, Phone: 469-419-4535 Email: [email protected] INTRODUCTION This lecture will discuss basic concepts, etiologic factors, pathologic features, pathogenesis, and clinicopathologic findings in the different types of interstitial lung diseases. It also will touch briefly on pulmonary vascular diseases. LEARNING OBJECTIVES: • Acute respiratory distress syndrome (ARDS) (BP, pp. 460-461) o Define ARDS and list common causes o Illustrate the mechanism of lung injury in ARDS and the role of proinflammatory and anti-inflammatory mediators o Describe the morphologic features of ARDS. Understand how they evolve, and know what happens if the patient survives. • Diffuse interstitial lung disease (BP, pp. 472-474, 480-482) o List the major forms of diffuse interstitial lung diseases discussed o Be able to identify classic morphologic features of common interstitial lung diseases. o Recognize the major causes of pneumoconioses (BP, pp. 474-478) o Delineate the gross and microscopic features resulting from exposure to coal dust, silica, organic/animal dust, and asbestos. o Sarcoidosis (BP, pp. 478-480) . Define sarcoidosis . List the most common organs involved, and describe the characteristic histologic lesion . Describe the radiographic, gross, and histologic appearance of lesions in the hilar lymph nodes and lungs • Define primary pulmonary hypertension. Recognize the characteristic histologic -

Idiopathic Bronchiolocentric Interstitial Pneumonia Samuel A
Idiopathic Bronchiolocentric Interstitial Pneumonia Samuel A. Yousem, MD, Sanja Dacic, MD, PhD Department of Pathology, University of Pittsburgh Medical Center, Presbyterian University Hospital, Pittsburgh, Pennsylvania plugs of fibromyxoid connective tissue, and poorly The authors report 10 patients with a distinctive formed granulomas (1–3). This triad of morphologic idiopathic bronchiolocentric interstitial pneumonia changes allows the pathologist to direct the pulmo- having some histologic similarities to hypersensitiv- nologist to question the patient for specific inhala- ity pneumonitis. Bronchiolocentric interstitial tional exposures. We have accumulated 10 cases pneumonia has a marked predilection for women that have very similar morphologic findings to hy- (80%) and occurs in middle age (40–50 years). Chest persensitivity pneumonitis, with the exclusion of radiographs and pulmonary function tests show in- interstitial granulomas, in which extensive investi- terstitial and restrictive lung disease, while the his- gations failed to reveal a cause for the inflamma- tologic appearance is that of a centrilobular inflam- tion. The clinicopathologic features of this idio- matory process with small airway fibrosis and pathic bronchiolocentric interstitial pneumonia are inflammation that radiates into the interstitium of the basis of this report which suggests a more ag- the distal acinus in a patchy fashion. Granulomas gressive and life threatening biologic behavior for are not identified. At a mean followup of approxi- bronchiolocentric interstitial pneumonia than nor- mately 4 years in nine patients, 33% of patients mally associated with hypersensitivity pneumonitis were dead of disease and 56% had persistent or or some of the other conditions in the histologic progressive disease suggesting a more aggressive differential diagnosis. course than hypersensitivity pneumonitis and non- specific interstitial pneumonia, the two major dis- ease processes in the differential diagnosis. -

Restrictive Lung Disease
Downloaded from https://academic.oup.com/ptj/article/48/5/455/4638136 by guest on 29 September 2021 RESTRICTIVE LUNG DISEASE WARREN M. GOLD, M.D. RESTRICTIVE LUNG DISEASE is a These disorders can be divided into two pattern of abnormal lung function defined by groups: extrapulmonary and pulmonary. a decrease in lung volume (Fig. I).1,2 The In extrapulmonary restriction, an abnormal total lung capacity is decreased and, in severe increase in the stiffness of the chest wall (kypho restrictive defects, all of the subdivisions of the scoliosis) restricts the lung volumes, as does total lung capacity including vital capacity, respiratory muscle weakness (poliomyelitis or functional residual capacity, and residual vol muscular dystrophy). These extrapulmonary ume are decreased. In mild or moderately se causes of pulmonary restriction are treated vere restrictive defects, the residual volume may be normal or slightly increased. CLINICAL DISORDERS CAUSING TABLE 1 RESTRICTIVE LUNG DISEASE CAUSES OF RESTRICTIVE LUNG DISEASE Restrictive lung disease is not a specific clin I. Extrapulmonary restriction 3 ical entity, but only one of several patterns of A. Chest wall stiffness (kyphoscoliosis) B. Respiratory-muscle weakness (muscular dystrophy)4 abnormal lung function. It is produced by a C. Pleural disease (pneumothorax)5 number of clinical disorders (see Table 1). II. Pulmonary restriction A. Surgical resection (pneumonectomy)6.7 Dr. Gold: Director, Pulmonary Laboratory and Re B. Tumor (bronchogenic carcinoma or metastatic tumor)8 search Associate in Cardiology (Pulmonary Physiology), C. Heart disease (hypertensive, arteriosclerotic, rheu Children's Hospital Medical Center; Associate in Pedi matic, congenital)9 atrics and Tutor in Medical Science, Harvard Medical 10 11 School, Boston, Massachusetts. -

Human Anatomy and Physiology
LECTURE NOTES For Nursing Students Human Anatomy and Physiology Nega Assefa Alemaya University Yosief Tsige Jimma University In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education 2003 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2003 by Nega Assefa and Yosief Tsige All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. Human Anatomy and Physiology Preface There is a shortage in Ethiopia of teaching / learning material in the area of anatomy and physicalogy for nurses. The Carter Center EPHTI appreciating the problem and promoted the development of this lecture note that could help both the teachers and students. -
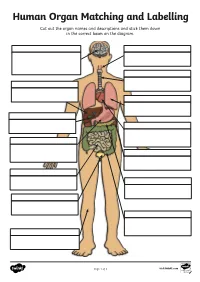
Human Organ Matching and Labelling Cut out the Organ Names and Descriptions and Stick Them Down in the Correct Boxes on the Diagram
Human Organ Matching and Labelling Cut out the organ names and descriptions and stick them down in the correct boxes on the diagram. Page 1 of 3 visit twinkl.com Maintains body temperature using Receives food from the oesophagus sweat and goosebumps. and begins to break it down with digestive juices (enzymes). Controls all of our necessary bodily functions, sends the Transports air from the nose and impulses which allow us to move mouth to the lungs. and enables you to think and learn. Pumps oxygenated blood around your body and receives de- oxygenated blood back. Filters water and salt out of your oesophagus blood and creates urine. bladder Makes bile for digestion, filters out toxins and regulates blood sugar. liver Produces enzymes necessary for large intestine digestion. gall bladder Digests food using enzymes and absorbs nutrients for the blood. kidneys Continues the digestion process, stomach absorbs as much water as possible and expels excess fibre and waste. heart Stores and concentrates bile pancreas produced by the liver. lungs Takes in oxygen, which reaches the blood via the heart. small intestine Stores urine so that we can decide trachea when we want to go to the toilet. skin Transports food and drink from the mouth to the stomach. brain Page 2 of 3 visit twinkl.com Answers brain oesophagus Controls all of our necessary bodily Transports food and drink from the functions, sends the impulses which mouth to the stomach. allow us to move and enables you to think and learn. trachea Transports air from the nose and liver mouth to the lungs. -

A Case Study in Organ Allocation Policy and Administrative Law
139 Journal of Health & Biomedical Law, XIV (2018) 139-148 © 2018 Journal of Health & Biomedical Law Suffolk University Law School THE LUNG LAWSUIT: A CASE STUDY IN ORGAN ALLOCATION POLICY AND ADMINISTRATIVE LAW Alexandra K. Glazier, J.D., M.P.H.* In November 2017, twenty-one-year-old Miriam Holman, was waiting for a lung transplant.1 Miriam was suffering from a rare form of pulmonary hypertension for which there is no medical therapy, and which is rapidly fatal without lung transplantation. Miriam was on an artificial lung machine in the ICU at Columbia Medical Center in New York where she was listed for organ transplantation.2 Organ allocation policies are, under federal law, designed to balance equity and utility principles based on medical criteria to rank order patients waiting for suitable organs to be available that are a biological match.3 Allocation policy therefore, incorporates factors such as (1) how critically ill the patient waiting is; (2) how long the patient has been waiting; and (3) for some organs allocation policies, the relative magnitude of the * Ms. Glazier is President and CEO of New England Donor Services, the two OPOs serving Connecticut, Maine, Massachusetts, New Hampshire, Rhode Island and Vermont, a member of the OPTN Board of Directors, previously chaired the OPTN Ethics Committee and has twice been appointed to the U.S. Secretary of HHS, Advisory Committee on Organ Transplantation. 1 Complaint at 18, Holman v. Secretary of HHS, No. 17-cv-09041 (S.D.N.Y. filed Nov. 11, 2017) (describing the plaintiff’s medical history). 2 Id. -

Biology - Student Learning Outcomes BIOL 090 Human Anatomy and 1
Biology - Student Learning Outcomes BIOL 090 Human Anatomy and 1. Explain how the major organ systems function. (ILO2, ILO5) Physiology for Health 2. Apply his/her knowledge of organ system function to solve problems based on Professionals materials and situations not covered directly in class. (ILO1, ILO2, ILO5) 3. Keep up-to-date with the materials that are covered in class. (ILO3, ILO4) BIOL 092 Microbiology For Advanced 1. understand research contributions of various scientists that have lead to the Placement of VN to RN development of modern day microbiology. (ILO4, ILO5) Nursing Students 2. understand the relationship between microbial morphology and function. (ILO2) 3. isolate pure microbial cultures using various aseptic techniques. (ILO2) 4. understand and explain microbial pathogenicty and etiology of disease. (ILO1) BIOL 100 Principles Of Biological 1. demonstrate an understanding of the steps of the scientific method. (ILO2) Science 2. communicate an understanding of the various patterns of inheritance of genetic traits. (ILO1, ILO2) 3. explain how the processes of natural selection influence evolution. (ILO1, ILO2) 4. perform lab activities properly, and correctly analyze lab data. (ILO1, ILO2) BIOL 120 General Zoology I 1. display oral communication effectiveness by doing an oral presentation of a research paper. (ILO1) 2. display the ability to show critical thinking by answering short essay type questions on exams. (ILO2) 3. display ability to understand written and illustrated information on the subject matter. (ILO4) 4. display an understanding of global impact on and by invertebrate animals. (ILO5) BIOL 122 General Zoology II 1. display oral communication effectiveness by an oral presentation of a research paper subject.