Foundational Model of Structural Connectivity in the Nervous System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Nerve Lesion in the Carpal Tunnel Syndrome
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.39.7.615 on 1 July 1976. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry, 1976, 39, 615-626 The nerve lesion in the carpal tunnel syndrome SYDNEY SUNDERLAND From the Department of Experimental Neurology, University of Melbourne, Parkville, Victoria, Australia SYNOPSIS The relative roles of pressure deformation and ischaemia in the production of compres- sion nerve lesions remain a controversial issue. This paper concerns the genesis of the structural changes which follow compression of the median nerve in the carpal tunnel. The initial lesion is an intrafunicular anoxia caused by obstruction to the venous return from the funiculi as the result of increased pressure in the tunnel. This leads to intrafunicular oedema and an increase in intrafunicular pressure which imperil and finally destroy nerve fibres by impairing their blood supply and by compression. The final outcome is the fibrous tissue replacement of the contents of the funiculi. Protected by copyright. In 1862 Waller described the motor, vasomotor, (Gasser, 1935; Allen, 1938; Bentley and Schlapp, and sensory changes which followed the com- 1943; Richards, 1951; Moldaver, 1954; Gelfan pression of nerves in his own arm. His account and Tarlov, 1956). carried no reference to the mechanism In the 1940s Weiss and his associates (Weiss, responsible for blocking conduction in the nerve 1943, 1944; Weiss and Davis, 1943; Weiss and fibres, presumably because he regarded it as Hiscoe, 1948), who were primarily concerned obvious that pressure was the offending agent. with the technical problem of uniting severed Interest in the effects of nerve compression nerves, observed that a divided nerve enclosed was not renewed until the 1920s when cuff or in a tightly fitting arterial sleeve became swollen tourniquet compression was used to produce a proximal and distal to the constriction. -

Deconstructing Spinal Interneurons, One Cell Type at a Time Mariano Ignacio Gabitto
Deconstructing spinal interneurons, one cell type at a time Mariano Ignacio Gabitto Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy under the Executive Committee of the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2016 © 2016 Mariano Ignacio Gabitto All rights reserved ABSTRACT Deconstructing spinal interneurons, one cell type at a time Mariano Ignacio Gabitto Abstract Documenting the extent of cellular diversity is a critical step in defining the functional organization of the nervous system. In this context, we sought to develop statistical methods capable of revealing underlying cellular diversity given incomplete data sampling - a common problem in biological systems, where complete descriptions of cellular characteristics are rarely available. We devised a sparse Bayesian framework that infers cell type diversity from partial or incomplete transcription factor expression data. This framework appropriately handles estimation uncertainty, can incorporate multiple cellular characteristics, and can be used to optimize experimental design. We applied this framework to characterize a cardinal inhibitory population in the spinal cord. Animals generate movement by engaging spinal circuits that direct precise sequences of muscle contraction, but the identity and organizational logic of local interneurons that lie at the core of these circuits remain unresolved. By using our Sparse Bayesian approach, we showed that V1 interneurons, a major inhibitory population that controls motor output, fractionate into diverse subsets on the basis of the expression of nineteen transcription factors. Transcriptionally defined subsets exhibit highly structured spatial distributions with mediolateral and dorsoventral positional biases. These distinctions in settling position are largely predictive of patterns of input from sensory and motor neurons, arguing that settling position is a determinant of inhibitory microcircuit organization. -
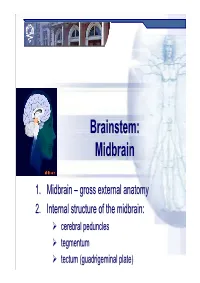
Brainstem: Midbrainmidbrain
Brainstem:Brainstem: MidbrainMidbrain 1.1. MidbrainMidbrain –– grossgross externalexternal anatomyanatomy 2.2. InternalInternal structurestructure ofof thethe midbrain:midbrain: cerebral peduncles tegmentum tectum (guadrigeminal plate) Midbrain MidbrainMidbrain –– generalgeneral featuresfeatures location – between forebrain and hindbrain the smallest region of the brainstem – 6-7g the shortest brainstem segment ~ 2 cm long least differentiated brainstem division human midbrain is archipallian – shared general architecture with the most ancient of vertebrates embryonic origin – mesencephalon main functions:functions a sort of relay station for sound and visual information serves as a nerve pathway of the cerebral hemispheres controls the eye movement involved in control of body movement Prof. Dr. Nikolai Lazarov 2 Midbrain MidbrainMidbrain –– grossgross anatomyanatomy dorsal part – tectum (quadrigeminal plate): superior colliculi inferior colliculi cerebral aqueduct ventral part – cerebral peduncles:peduncles dorsal – tegmentum (central part) ventral – cerebral crus substantia nigra Prof. Dr. Nikolai Lazarov 3 Midbrain CerebralCerebral cruscrus –– internalinternal structurestructure CerebralCerebral peduncle:peduncle: crus cerebri tegmentum mesencephali substantia nigra two thick semilunar white matter bundles composition – somatotopically arranged motor tracts: corticospinal } pyramidal tracts – medial ⅔ corticobulbar corticopontine fibers: frontopontine tracts – medially temporopontine tracts – laterally -

The Brain Stem Medulla Oblongata
Chapter 14 The Brain Stem Medulla Oblongata Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Central sulcus Parietal lobe • embryonic myelencephalon becomes Cingulate gyrus leaves medulla oblongata Corpus callosum Parieto–occipital sulcus Frontal lobe Occipital lobe • begins at foramen magnum of the skull Thalamus Habenula Anterior Epithalamus commissure Pineal gland • extends for about 3 cm rostrally and ends Hypothalamus Posterior commissure at a groove between the medulla and Optic chiasm Mammillary body pons Cerebral aqueduct Pituitary gland Fourth ventricle Temporal lobe • slightly wider than spinal cord Cerebellum Midbrain • pyramids – pair of external ridges on Pons Medulla anterior surface oblongata – resembles side-by-side baseball bats (a) • olive – a prominent bulge lateral to each pyramid • posteriorly, gracile and cuneate fasciculi of the spinal cord continue as two pair of ridges on the medulla • all nerve fibers connecting the brain to the spinal cord pass through the medulla • four pairs of cranial nerves begin or end in medulla - IX, X, XI, XII Medulla Oblongata Associated Functions • cardiac center – adjusts rate and force of heart • vasomotor center – adjusts blood vessel diameter • respiratory centers – control rate and depth of breathing • reflex centers – for coughing, sneezing, gagging, swallowing, vomiting, salivation, sweating, movements of tongue and head Medulla Oblongata Nucleus of hypoglossal nerve Fourth ventricle Gracile nucleus Nucleus of Cuneate nucleus vagus -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Nerve and Nerve Injuries” Sunderland : 50 Years Later
2019 Nerve and Nerve Injuries” Sunderland : 50 years later Faye Chiou Tan, MD Professor, Dir. EDX, H. Ben Taub PMR, Baylor College of Medicine Chief PMR, Dir. EDX, Harris Health System 2019 Financial Disclosure • Elsevier Book Royalties for “EMG Secrets” textbook • Revance, consultation panel 2019 Warning Videotaping or taking pictures of the slides associated with this presentation is prohibited. The information on the slides is copyrighted and cannot be used without permission and author attribution. Introduction – Sydney Sunderland was Professor of Experimental Neurology at the University of Melbourne. – His textbook “Nerve and Nerve lnjuries” published in 1968 is no longer in print (copies $1000 on the internet) – Here is a review as relates to new technology: Ultrahigh frequency musculoskeletal ultrasound Part I – I. Anatomic and physiologic features of A. Peripheral nerve fibers B. Peripheral nerve trunks I.A. Peripheral nerve fibers – Axoplasm – Increased flow of cytoplasm from cell body into axons during electrical stimulation (Grande and Richter 1950) – Although overall proximal to distal axoplasmic flow, the pattern of streaming in the axon is bidirectional and faster (up to 3-7 cm/day) (Lubinska 1964). I. A. Peripheral nerve fibers – Sheath – Myelinated – Length of internode elongates with growth (Vizoso and Young 1948, Siminoff 1965) – In contrast, remyelination in adults produce short internodes of same length (Leegarrd 1880, Young 1945,…) – Incisures of Schmidt-Lantermann are clefts conical clefts that open when a nerve trunk is stretched thereby preventing distortion of myelin. (Glees, 1943) Schmidt-Lantermann Clefts Sunderland S. Nerve and Nerve Injuries, Sunderland, Livingstone,LTD, Edinburgh/London, 1968, p. 8 I. A. -

Review of Spinal Cord Basics of Neuroanatomy Brain Meninges
Review of Spinal Cord with Basics of Neuroanatomy Brain Meninges Prof. D.H. Pauža Parts of Nervous System Review of Spinal Cord with Basics of Neuroanatomy Brain Meninges Prof. D.H. Pauža Neurons and Neuroglia Neuron Human brain contains per 1011-12 (trillions) neurons Body (soma) Perikaryon Nissl substance or Tigroid Dendrites Axon Myelin Terminals Synapses Neuronal types Unipolar, pseudounipolar, bipolar, multipolar Afferent (sensory, centripetal) Efferent (motor, centrifugal, effector) Associate (interneurons) Synapse Presynaptic membrane Postsynaptic membrane, receptors Synaptic cleft Synaptic vesicles, neuromediator Mitochondria In human brain – neurons 1011 (100 trillions) Synapses – 1015 (quadrillions) Neuromediators •Acetylcholine •Noradrenaline •Serotonin •GABA •Endorphin •Encephalin •P substance •Neuronal nitric oxide Adrenergic nerve ending. There are many 50-nm-diameter vesicles (arrow) with dark, electron-dense cores containing norepinephrine. x40,000. Cell Types of Neuroglia Astrocytes - Oligodendrocytes – Ependimocytes - Microglia Astrocytes – a part of hemoencephalic barrier Oligodendrocytes Ependimocytes and microglial cells Microglia represent the endogenous brain defense and immune system, which is responsible for CNS protection against various types of pathogenic factors. After invading the CNS, microglial precursors disseminate relatively homogeneously throughout the neural tissue and acquire a specific phenotype, which clearly distinguish them from their precursors, the blood-derived monocytes. The ´resting´ microglia -

Cajal's Law of Dynamic Polarization: Mechanism and Design
philosophies Article Cajal’s Law of Dynamic Polarization: Mechanism and Design Sergio Daniel Barberis ID Facultad de Filosofía y Letras, Instituto de Filosofía “Dr. Alejandro Korn”, Universidad de Buenos Aires, Buenos Aires, PO 1406, Argentina; sbarberis@filo.uba.ar Received: 8 February 2018; Accepted: 11 April 2018; Published: 16 April 2018 Abstract: Santiago Ramón y Cajal, the primary architect of the neuron doctrine and the law of dynamic polarization, is considered to be the founder of modern neuroscience. At the same time, many philosophers, historians, and neuroscientists agree that modern neuroscience embodies a mechanistic perspective on the explanation of the nervous system. In this paper, I review the extant mechanistic interpretation of Cajal’s contribution to modern neuroscience. Then, I argue that the extant mechanistic interpretation fails to capture the explanatory import of Cajal’s law of dynamic polarization. My claim is that the definitive formulation of Cajal’s law of dynamic polarization, despite its mechanistic inaccuracies, embodies a non-mechanistic pattern of reasoning (i.e., design explanation) that is an integral component of modern neuroscience. Keywords: Cajal; law of dynamic polarization; mechanism; utility; design 1. Introduction The Spanish microanatomist and Nobel laureate Santiago Ramón y Cajal (1852–1934), the primary architect of the neuron doctrine and the law of dynamic polarization, is considered to be the founder of modern neuroscience [1,2]. According to the neuron doctrine, the nerve cell is the anatomical, physiological, and developmental unit of the nervous system [3,4]. To a first approximation, the law of dynamic polarization states that nerve impulses are exactly polarized in the neuron; in functional terms, the dendrites and the cell body work as a reception device, the axon works as a conduction device, and the terminal arborizations of the axon work as an application device. -
The Churchlands' Neuron Doctrine: Both Cognitive and Reductionist
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/231998559 The Churchlands' neuron doctrine: Both cognitive and reductionist Article in Behavioral and Brain Sciences · October 1999 DOI: 10.1017/S0140525X99462193 CITATIONS READS 2 20 1 author: John Sutton Macquarie University 139 PUBLICATIONS 1,356 CITATIONS SEE PROFILE All content following this page was uploaded by John Sutton on 06 August 2014. The user has requested enhancement of the downloaded file. All in-text references underlined in blue are added to the original document and are linked to publications on ResearchGate, letting you access and read them immediately. The Churchlands’ neuron doctrine: both cognitive and reductionist John Sutton Macquarie University, Sydney, NSW 2109, Australia Behavioral and Brain Sciences, 22 (1999), 850-1. [email protected] http://johnsutton.net/ Commentary on Gold & Stoljar, ‘A Neuron Doctrine in the Philosophy of Neuroscience’, Behavioral and Brain Sciences, 22 (1999), 809-869: full paper and commentaries online at: http://www.stanford.edu/~paulsko/papers/GSND.pdf Abstract: According to Gold and Stoljar, one cannot both consistently be reductionist about psychoneural relations and invoke concepts developed in the psychological sciences. I deny the utility of their distinction between biological and cognitive neuroscience, suggesting that they construe biological neuroscience too rigidly and cognitive neuroscience too liberally. Then I reject their characterization of reductionism: reductions need not go down past neurobiology straight to physics, and cases of partial, local reduction are not neatly distinguishable from cases of mere implementation. Modifying the argument from unification-as-reduction, I defend a position weaker than the radical, but stronger than the trivial neuron doctrine. -

Richard Scheller and Thomas Südhof Receive the 2013 Albert Lasker Basic Medical Research Award
Richard Scheller and Thomas Südhof receive the 2013 Albert Lasker Basic Medical Research Award Jillian H. Hurst J Clin Invest. 2013;123(10):4095-4101. https://doi.org/10.1172/JCI72681. News Neural communication underlies all brain activity. It governs our thoughts, feelings, sensations, and actions. But knowing the importance of neural communication does not answer a central question of neuroscience: how do individual neurons communicate? We know that communication between two neurons occurs at specialized cell junctions called synapses, at which two communicating neurons are separated by the synaptic cleft. The presynaptic neuron releases chemicals, known as neurotransmitters, into the synaptic cleft in which neurotransmitters bind to receptors on the surface of the postsynaptic neuron. Neurotransmitter release occurs in response to an action potential within the sending neuron that induces depolarization of the nerve terminal and causes an influx of calcium. Calcium influx triggers the release of neurotransmitters through a specialized form of exocytosis in which neurotransmitter-filled vesicles fuse with the plasma membrane of the presynaptic nerve terminal in a region known as the active zone, spilling neurotransmitter into the synaptic cleft. By the 1950s, it was clear that brain function depended on chemical neurotransmission; however, the molecular activities that governed neurotransmitter release were virtually unknown until the early 1990s. This year, the Lasker Foundation honors Richard Scheller (Genentech) and Thomas Südhof (Stanford University School of Medicine) for their “discoveries concerning the molecular machinery and regulatory mechanisms that underlie the rapid release of neurotransmitters.” Over the course of two decades, Scheller […] Find the latest version: https://jci.me/72681/pdf News Richard Scheller and Thomas Südhof receive the 2013 Albert Lasker Basic Medical Research Award Neural communication underlies all Setting the stage um-driven action potentials elicited neu- brain activity. -

High-Yield Neuroanatomy
LWBK110-3895G-FM[i-xviii].qxd 8/14/08 5:57 AM Page i Aptara Inc. High-Yield TM Neuroanatomy FOURTH EDITION LWBK110-3895G-FM[i-xviii].qxd 8/14/08 5:57 AM Page ii Aptara Inc. LWBK110-3895G-FM[i-xviii].qxd 8/14/08 5:57 AM Page iii Aptara Inc. High-Yield TM Neuroanatomy FOURTH EDITION James D. Fix, PhD Professor Emeritus of Anatomy Marshall University School of Medicine Huntington, West Virginia With Contributions by Jennifer K. Brueckner, PhD Associate Professor Assistant Dean for Student Affairs Department of Anatomy and Neurobiology University of Kentucky College of Medicine Lexington, Kentucky LWBK110-3895G-FM[i-xviii].qxd 8/14/08 5:57 AM Page iv Aptara Inc. Acquisitions Editor: Crystal Taylor Managing Editor: Kelley Squazzo Marketing Manager: Emilie Moyer Designer: Terry Mallon Compositor: Aptara Fourth Edition Copyright © 2009, 2005, 2000, 1995 Lippincott Williams & Wilkins, a Wolters Kluwer business. 351 West Camden Street 530 Walnut Street Baltimore, MD 21201 Philadelphia, PA 19106 Printed in the United States of America. All rights reserved. This book is protected by copyright. No part of this book may be reproduced or transmitted in any form or by any means, including as photocopies or scanned-in or other electronic copies, or utilized by any information storage and retrieval system without written permission from the copyright owner, except for brief quotations embodied in critical articles and reviews. Materials appearing in this book prepared by individuals as part of their official duties as U.S. government employees are not covered by the above-mentioned copyright. To request permission, please contact Lippincott Williams & Wilkins at 530 Walnut Street, Philadelphia, PA 19106, via email at [email protected], or via website at http://www.lww.com (products and services). -

From the Neuron Doctrine to Neural Networks
LINK TO ORIGINAL ARTICLE LINK TO INITIAL CORRESPONDENCE other hand, one of my mentors, David Tank, argued that for a true understanding of a On testing neural network models neural circuit we should be able to actu- ally build it, which is a stricter definition Rafael Yuste of a successful theory (D. Tank, personal communication) Finally, as mentioned in In my recent Timeline article, I described existing neural network models have enough the Timeline article, one will also need to the emergence of neural network models predictive value to be considered valid or connect neural network models to theories as an important paradigm in neuroscience useful for explaining brain circuits.” (REF. 1)). and facts at the structural and biophysical research (From the neuron doctrine to neu- There are many exciting areas of progress levels of neural circuits and to those in cog- ral networks. Nat. Rev. Neurosci. 16, 487–497 in current neuroscience detailing phenom- nitive sciences as well, for proper ‘scientific (2015))1. In his correspondence (Neural enology that is consistent with some neural knowledge’ to occur in the Kantian sense. networks in the future of neuroscience network models, some of which I tried to research. Nat. Rev. Neurosci. http://dx.doi. summarize and illustrate, but at the same Rafael Yuste is at the Neurotechnology Center and org/10.1038/nrn4042 (2015))2, Rubinov time we are still far from a rigorous demon- Kavli Institute of Brain Sciences, Departments of provides some thoughtful comments about stration of any neural network model with Biological Sciences and Neuroscience, Columbia University, New York, New York 10027, USA.