Perplexing Case of Wilkie's Syndrome: a Rare Presentation in a Young Patient
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From Nutcracker Phenomenon to Nutcracker Syndrome: a Pictorial Review
diagnostics Review From Nutcracker Phenomenon to Nutcracker Syndrome: A Pictorial Review Antonio Granata 1,†, Giulio Distefano 2,*,† , Alessio Sturiale 1, Michele Figuera 3, Pietro Valerio Foti 2, Stefano Palmucci 2 and Antonio Basile 2 1 Nephrology and Dialysis Unit, “Cannizzaro” Hospital, 95026 Catania, Italy; [email protected] (A.G.); [email protected] (A.S.) 2 Radiology Unit I, Department of Medical Surgical Sciences and Advanced Technologies “GF Ingrassia”, University Hospital “Policlinico—San Marco”, University of Catania, 95123 Catania, Italy; [email protected] (P.V.F.); [email protected] (S.P.); [email protected] (A.B.) 3 Radiology Unit II, University Hospital “Policlinico—San Marco”, 95123 Catania, Italy; micfi[email protected] * Correspondence: [email protected]; Tel.: +39-3385020778 † These authors contributed equally to this work. Abstract: Left renal vein (LRV) entrapment, also known as nutcracker phenomenon if it is asymp- tomatic, is characterized by abnormality of outflow from the LRV into the inferior vena cava (IVC) due to extrinsic LRV compression, often accompanied by demonstrable lateral (hilar) dilatation and medial (mesoaortic) stenosis. Nutcracker syndrome, on the other hand, includes a well-defined set of symptoms, and the severity of these clinical manifestations is related to the severity of anatomic and hemodynamic findings. With the aim of providing practical guidance for nephrologists and radiolo- gists, we performed a review of the literature through the PubMed database, and we commented on the definition, the main clinical features, and imaging pattern of this syndrome; we also researched the main therapeutic approaches validated in the literature. Finally, from the electronic database of our institute, we have selected some characteristic cases and we have commented on the imaging pattern of this disease. -

Diagnosis and F이low-Up of a Case of Nutcracker Syndrome with MR Angiography
대 한 방 사 선 의 학 회 지 1993 ; 29 (3) : 426~429 Journ al of Korea n Radiological Society, Ma y, 1993 Diagnosis and F이low-up of a Case of Nutcracker Syndrome with MR Angiography Gwy Suk Seo, M.D., Hyo Keun Lim, M.D., Sang Hoon Bae, M.D., Kyung Hwan Lee, M.D., Dong Wan Chae, M.D.*, Hong Rae Cho, M.D.**, Ku Sub Yun, M.D. Departmeη t 01 Diagnostic R adiology, Hallym Uηzveγsi ty College 01 Mediciηe - Abstract - A case of nutcracker syndrome which was initially diagnosed by magnetic resonance angiography (MRA) is reported. On preoperative MRA in an 18-year-old male patient with gross hematuria, left renal vein was oblit erated at the level of superior mesenteric arteη and there was no connection with inferior vena cava. The fol low-up MRA after surgical coπection with external prosthesis demonstrated entire course of left renal vein without evidence of obstruction which might suggest a possible usage of MRA for a non-invasive diagnosis of nutcracker syndrome. Index Words: Magnetic resonance angiography 966.1299 Renal vein, stenosis 966.78 So-called the nutcracker syndrome, which We described a patient who had a clinical shows variable degree of hematuria mairùy be finding of nutcracker syndrome and the m ‘ jor cause of compression of the left renal vein be role of MRA in the diagnosis and the postoper tween abdorninal aorta and superior mesenteric ative follow-up. artery, is quite a nuisant disease entity in terms of imaging diagnosis (1 -3). Although left renal CASE REPORT venogram with pressure measurement was re garded as a g이d standard of the diagnosis, A 18-year-old male patient who had a re arteriogram or ultrasonogram is now regarded current massive gross hematuria for six months as a helpful, but no longer a valuable diagnostic was adrnitted. -

Mixed Nutcracker Syndrome with Left Renal Vein Duplication: a Severe and Exceptional Presentation in an 18‑Year‑Old Boy
[Downloaded free from http://www.urologyannals.com on Sunday, April 26, 2015, IP: 41.68.70.140] Case Report Mixed nutcracker syndrome with left renal vein duplication: A severe and exceptional presentation in an 18‑year‑old boy Faouzi Mallat, Wissem Hmida, Mouna Ben Othmen, Faouzi Mosbah Department of Urology, Hospital of Sahloul, Sousse, Tunisia Abstract The nutcracker syndrome (NCS) is rare and often misdiagnosed because it embraces an extended non-pathognomonic spectrum of symptoms that imply a difficult diagnosis. Ultimately it may be associated with substantial morbidity and even life‑threatening events. Mixed NCS with renal vein duplication is an exceptional variety, have previously been reported to the best of our knowledge. We report a rare case of an 18-year-old boy who presented with a long history of abdominal, pelvic and left flank pain, fatigue and higher bilateral varicocele. Computed tomographic angiography, Doppler ultrasonography and venography were performed revealed left renal vein duplication with dilated retroaortic and preaortic branchs, entrapped respectively between the aorta and the vertebral column and in the aortico-mesenteric space, with extensive and complex varices of the deep pelvic venous plexus; promoting the mixed renal NCS. Auto transplantation of the left kidney was suggested, but refused by the patient; and only the varicocele was managed. The patient is still suffering from his severe initial symptoms. Diagnosis is difficult and should be considered in patients with inexplicable flank or abdominal pain. Our purpose is to raise clinician’s awareness for this condition so that they will be more likely to diagnose it. This will facilitate prompt diagnosis and treatment. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub
US 2010O2.10567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub. Date: Aug. 19, 2010 (54) USE OF ATUFTSINASATHERAPEUTIC Publication Classification AGENT (51) Int. Cl. A638/07 (2006.01) (76) Inventor: Dorian Bevec, Germering (DE) C07K 5/103 (2006.01) A6IP35/00 (2006.01) Correspondence Address: A6IPL/I6 (2006.01) WINSTEAD PC A6IP3L/20 (2006.01) i. 2O1 US (52) U.S. Cl. ........................................... 514/18: 530/330 9 (US) (57) ABSTRACT (21) Appl. No.: 12/677,311 The present invention is directed to the use of the peptide compound Thr-Lys-Pro-Arg-OH as a therapeutic agent for (22) PCT Filed: Sep. 9, 2008 the prophylaxis and/or treatment of cancer, autoimmune dis eases, fibrotic diseases, inflammatory diseases, neurodegen (86). PCT No.: PCT/EP2008/007470 erative diseases, infectious diseases, lung diseases, heart and vascular diseases and metabolic diseases. Moreover the S371 (c)(1), present invention relates to pharmaceutical compositions (2), (4) Date: Mar. 10, 2010 preferably inform of a lyophilisate or liquid buffersolution or artificial mother milk formulation or mother milk substitute (30) Foreign Application Priority Data containing the peptide Thr-Lys-Pro-Arg-OH optionally together with at least one pharmaceutically acceptable car Sep. 11, 2007 (EP) .................................. O7017754.8 rier, cryoprotectant, lyoprotectant, excipient and/or diluent. US 2010/0210567 A1 Aug. 19, 2010 USE OF ATUFTSNASATHERAPEUTIC ment of Hepatitis BVirus infection, diseases caused by Hepa AGENT titis B Virus infection, acute hepatitis, chronic hepatitis, full minant liver failure, liver cirrhosis, cancer associated with Hepatitis B Virus infection. 0001. The present invention is directed to the use of the Cancer, Tumors, Proliferative Diseases, Malignancies and peptide compound Thr-Lys-Pro-Arg-OH (Tuftsin) as a thera their Metastases peutic agent for the prophylaxis and/or treatment of cancer, 0008. -

Acute Pulmonary Thromboembolism in a Patient with Nutcracker Syndrome and Antiphospholipid Syndrome
CASE REPORT Acute Pulmonary Thromboembolism in a Patient with Nutcracker Syndrome and Antiphospholipid Syndrome Takayasu Ito,1 MD, Ryuji Okamoto,1 MD, Akimasa Matsuda,1 MD, Yoshito Ogihara,1 MD, Norikazu Yamada,1,2 MD and Masaaki Ito,1 MD Summary Nutcracker syndrome (NCS), which is defined as compression of the left renal vein between the aorta and the superior mesenteric artery, is usually benign and self-limiting. Long-term renal venous retention increases the risk of renal vein thrombosis. However, NCS rarely develops into isolated thrombosis of the left renal vein; the reason for this process remains unknown. We describe a young man with antiphospholipid syndrome, who developed overt pulmonary thromboembolism due to an isolated thrombus in the left renal vein. Complicating antiphospholipid syndrome might trigger acute pulmonary thromboembolism (APTE) in patients with NCS. To the best of our knowledge, this is the first report of APTE arising due to isolated left renal vein thrombosis in patients with NCS. (Int Heart J 2020; 61: 856-858) Key words: Compression of the left renal vein, Renal vein thrombosis, Systemic lupus erythematosus utcracker syndrome (NCS) is defined as compres- poxic changes were evident in his fingers and toes (Figure sion of the left renal vein, which usually lies be- B). Contrast computed tomography (CT) showed left renal N tween the superior mesenteric artery and the vein thrombosis and a minor APTE in the right lower aorta. If the angle between these two arteries is < 35°, lung field (Figure C), but there was no evidence of throm- symptoms, such as hematuria, proteinuria, flank pain, pel- bus in the right and left heart chambers. -

An Incidental Detection of Nutcracker Phenomenon in an Adolescent with Transient High Blood Pressure: a Case Report
Case Reports in Clinical Medicine, 2016, 5, 431-436 http://www.scirp.org/journal/crcm ISSN Online: 2325-7083 ISSN Print: 2325-7075 An Incidental Detection of Nutcracker Phenomenon in an Adolescent with Transient High Blood Pressure: A Case Report Min Cai1, Haibin Chen2, Haixiong Wang3, Hong Zhang3, Guisheng Feng1, Xiaohong Zhang4, Jian Chen4, Jiyun Du3 1Department of Nuclear Medicine, Shanxi Provincial People’s Hospital, Taiyuan, China 2Department of Anorectal Surgery, Shanxi Hospital of Integrated Traditional and Western Medicine, Taiyuan, China 3Department of Cardiology, Shanxi Provincial People’s Hospital, Taiyuan, China 4Department of Ultrasound, Shanxi Provincial People’s Hospital, Taiyuan, China How to cite this paper: Cai, M., Chen, Abstract H.B., Wang, H.X., Zhang, H., Feng, G.S., Zhang, X.H., Chen, J. and Du, J.Y. (2016) Nutcracker phenomenon (NCP) is caused by a compression of left renal vein be- An Incidental Detection of Nutcracker tween aorta and superior mesenteric artery. The traditional clinical manifestations of Phenomenon in an Adolescent with Tran- nutcracker syndrome is usually accompanied with abdominal pain, hematuria, or- sient High Blood Pressure: A Case Report. Case Reports in Clinical Medicine, 5, 431- thostatic proteinuria, and varicocele formation, however, hypertension is rarely re- 436. ported as main clinical feature. We describe a male adolescent with manifestation of http://dx.doi.org/10.4236/crcm.2016.511060 hypertension who was identified as NCP. Renal ultrasound and computed tomogra- Received: October 19, 2016 phy angiography have provided evidences of left renal vein dilatation, probably due Accepted: November 1, 2016 to the compression through the decreased angle between aorta and superior mesen- Published: November 4, 2016 teric artery. -

Nutcracker Syndrome Due to Chronic Aortic Dissection
Nutcracker Syndrome due to Chronic Aortic Dissection YUKI ICHIHARA1, Takashi Azuma1, Satoshi Saito1, and Hiroshi Niinami1 1Tokyo Women's Medical University November 24, 2020 Abstract Nutcracker syndrome (NCS) is known as a status of compression of the left renal vein (LRV) between the abdominal aorta and superior mesenteric artery (SMA). We here report a case of NCS in Marfan patients with type B aortic dissection who presented with sudden gross hematuria. Computed tomography revealed the compression of the LRV sandwiched between the SMA and the dilated dissecting abdominal aorta. The compression was released after surgical intervention and the hematuria was promptly recovered. This report highlights that NCS should be considered as a differential diagnosis of unexplained hematuria in patients with the chronic dissecting aorta. Nutcracker Syndrome due to Chronic Aortic Dissection Yuki Ichihara, MD, PhD+ , Takashi Azuma, MD, PhD, Satoshi Saito, MD, PhD, Hiroshi Niinami, MD, PhD Department of Cardiovascular Surgery, Tokyo Women's Medical University + Corresponding author: Yuki Ichihara 8-1, Kawada-cho, Shinjuku-ku, Tokyo, Japan, 162-8666 Tel:(+81) 3-3353-8111 Fax:(+81) 3-3356-0441 Email:[email protected] Word count:500/500 Abstract Nutcracker syndrome (NCS) is known as a status of compression of the left renal vein (LRV) between the abdominal aorta and superior mesenteric artery (SMA). We here report a case of NCS in Marfan patients with type B aortic dissection who presented with sudden gross hematuria. Computed tomography revealed the compression of the LRV sandwiched between the SMA and the dilated dissecting abdominal aorta. The compression was released after surgical intervention and the hematuria was promptly recovered. -

The 60Th Annual Meeting of the Japanese Society of Neurology Accepted Abstracts from Overseas
The 60th Annual Meeting of the Japanese Society of Neurology Accepted Abstracts from Overseas ★If you are nominated as a presenter at the "Nominees for Best Presentation Award for the International Participants," please prepare for the poster presentation in addition to your general oral/poster presentation. Nominees Presentati Present Travel Submitted Presentation for the on Title Start End Room ation Session Title Order Grant Abstract ID Date Award Number Style Winner (★) The association of white matter hyperintensity and functional outcomes in minor stroke and Poster 1000012 Pe-023-5 5/23(Thu) 17:50 19:05 Poster Cerebrovascular disease (clinical research5) 5 TG TIA Session Poster 1000014 Pe-011-4 Urinary calculi as acute stroke risk factor 5/22(Wed) 17:20 18:35 Poster Cerebrovascular disease (Biomaker) 4 TG Session Poster Parkinsonism and Related disorders 1000020 Pe-051-3 Phantom Bedside Intruder in Parkinsons Disease 5/24(Fri) 16:00 17:15 Poster 3 Session (cognition1) 1000021 withdrawn Homozygous splice-site mutation c.78+5 G>A in PMP22 causes congenital hypomyelinating Poster 1000024 Pe-075-1 5/25(Sat) 13:15 14:30 Poster Peripheral neuropathy (Miscellaneous3) 1 TG neuropathy Session 1000025 O-24-2 Clinical and genetic spectrum of sarcoglycanopathies in a large cohort of Chinese patients 5/23(Thu) 09:45 10:45 Room 14 Oral Myopathy1 2 TG Effects of Multi-Session rTMS on Motor Control and Spontaneous Brain Activity in MSA: A Pilot Poster Parkinsonism and Related disorders (Clinical 1000027 Pe-027-1 5/23(Thu) 17:50 19:05 Poster 1 TG -

Pulsatile Mass Sensation with Intense Abdominal Pain; Atypical
Signature: © Pol J Radiol, 2016; 81: 507-509 DOI: 10.12659/PJR.898166 CASE REPORT Received: 2016.02.22 Accepted: 2016.04.10 Pulsatile Mass Sensation with Intense Abdominal Pain; Published: 2016.10.28 Atypical Presentation of the Nutcracker Syndrome Authors’ Contribution: Ahmet Aslan1,2ABCDEF, Hakan Barutca1ABDF, Cemal Kocaaslan3BCDE, A Study Design 4BCDE 1CDE B Data Collection Süleyman Orman , Sinan Şahin C Statistical Analysis 1 D Data Interpretation Department of Radiology, Dr. Siyami Ersek Thoracic and Cardiovascular Surgery Training and Research Hospital, Kadiköy, E Manuscript Preparation Istanbul, Turkey 2 F Literature Search Department of Radiology, Ümraniye Training and Research Hospital, Ümraniye, Istanbul, Turkey (Present) 3 G Funds Collection Department of Cardiovascular Surgery, Dr. Siyami Ersek Thoracic and Cardiovascular Surgery Training and Research Hospital, Kadiköy, Istanbul, Turkey 4 Department of Gastrointestinal Surgery, Göztepe Training and Research Hospital, Medical School of Istanbul Medeniyet University, Kadiköy, Istanbul, Turkey Author’s address: Ahmet Aslan, Department of Radiology, Ümraniye Training and Research Hospital, 34760, Ümraniye, Istanbul, Turkey, e-mail: [email protected] Summary Background: Patients with Nutcracker syndrome generally present with nonspecific abdominal pain, with the left renal vein (LRV) lodged between the aorta and the superior mesenteric artery. In rare cases this can result in atypical gastrointestinal symptoms, making the diagnosis of Nutcracker syndrome challenging. Case Report: A 28-year-old female patient presented with complaints of severe abdominal pain and palpable pulsatile abdominal mass located in the left epigastric area. Computed tomography angiography revealed that the LRV was lodged in the aortomesenteric region with a dilated left ovarian vein and pelvic varicose veins. -
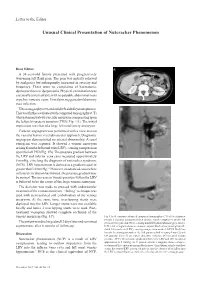
Unusual Clinical Presentation of Nutcracker Phenomenon
Nutcracker Syndrome: Unusual Presentation—Ai Peng Tan et al 470 Letter to the Editor Unusual Clinical Presentation of Nutcracker Phenomenon Dear Editor, A 34-year-old female presented with progressively worsening left flank pain. The pain was initially relieved by analgesics but subsequently increased in severity and frequency. There were no complaints of haematuria, dysmenorrhoea or dyspareunia. Physical examination was essentially unremarkable with no palpable abdominal mass or pelvic varicose veins. Urinalysis suggested mild urinary tract infection. Ultrasonography revealed mild left-sided hydronephrosis. This was further evaluated with computed tomography (CT) which demonstrated a vascular aneurysm compressing upon the left pelvi-ureteric junction (PUJ) (Fig. 1A). The initial impression was that of a large left renal artery aneurysm. Catheter angiogram was performed with a view to treat the vascular lesion via endovascular approach. Diagnostic angiogram demonstrated no arterial abnormality. A renal venogram was acquired. It showed a venous aneurysm arising from the left renal vein (LRV), causing compression upon the left PUJ (Fig. 1B). The pressure gradient between the LRV and inferior vena cava measured approximately 5 mmHg, clinching the diagnosis of nutcracker syndrome (NCS). LRV hypertension is defined as a gradient equal or greater than 3.0 mm Hg.1,2 However, in advanced cases where collateral circulation has formed, the pressure gradient may be normal. The increase in venous pressure within the LRV is believed to be the cause of the large venous aneurysm. The decision was made to proceed with endovascular treatment of the venous aneurysm. “Jailing” technique was used with stent-assisted coil embolisation of the venous aneurysm. -

Nutcracker Syndrome Combined with Superior Mesenteric Artery Syndrome in a Pediatric Patient: a Case Report
Case report Child Kidney Dis 2018;22:75-80 ISSN 2384-0242 (print) DOI: https://doi.org/10.3339/jkspn.2018.22.2.75 ISSN 2384-0250 (online) Nutcracker Syndrome combined with Superior Mesenteric Artery Syndrome in a Pediatric Patient: A Case Report Kyung Wook Min, M.D. Nutcracker syndrome is a phenomenon that the left renal vein (LRV) is pressed bet- Oh Kyung Lee, M.D. ween the superior mesenteric artery (SMA) and the aorta. Clinical characteristics Mi Kyung Kim, M.D. include gross or microscopic hematuria, orthostatic proteinuria, abdominal pain, and back pain. It occurs due to LRV squeezing caused by narrowed aortomesenteric Department of Pediatrics, Presbyterian angle. SMA syndrome is a disease that the third part of the duodenum is prone to Medical Center, Jeonju, Korea intestinal obstruction by narrowed angle between the SMA and the abdominal aorta. Clinical symptoms include postprandial abdominal distension, epigastric pain, nausea, and vomiting. SMA syndrome and nutcracker syndrome have com- Corresponding author: mon features that result from narrowed aortomesenteric angle. However, it is very Oh Kyung Lee, MD. Department of Pediatrics, Presbyterian rare for both syndromes to occur simultaneously, so the two syndromes are re- Medical Center, 365 Seowon-ro, Wansan- garded as separate diseases. This is a report on a case of nutcracker syndrome with gu, Jeonju 54987, Korea SMA syndrome in a child who presented gross hematuria, recurrent abdominal Tel: +82-63-230-1390 pain and vomiting. To our knowledge, nutcracker syndrome simultaneous with Fax: +82-63-230-1396 SMA syndrome has not been previously reported in pediatric patient, especially E-mail: [email protected] with an exhibition of gross hematuria. -

Diagnosis and Treatment of Renovascular Disease in Children Premal A
Diagnosis and Treatment of Renovascular Disease in Children Premal A. Patel, BSc (Hons), MBBS, MRCS, FRCR,* and Jelena Stojanovic, MD, FRCPCH† Introduction recommend regular BP screening in children.2 The recommen- dations state every child above 3 years of age should have BP s with other forms of endovascular treatments, renovas- measured at each clinical setting,3 and a recent large study dem- A cular intervention has an established role in adults. onstrated 36% of screened children had elevated BP.4 However, There is an increasing volume of data supporting its use in in practice only a third of children attending a hospital appoint- children. In particular, it is useful in the diagnosis and man- ment have their BP measured.5 Pediatric hypertension is defined agement of pediatric hypertension which often has a renovas- as systolic BP >95th percentile for age, sex, and height.6 Hyper- cular cause. The popularity of endovascular techniques to tension is diagnosed on 24-hour ambulatory BP monitoring for treat angiomylipomas as well as nutcracker syndrome (NS) is children above the age of 5 years. In younger children, at least 2 growing, and many groups are actively studying the effects of consecutive sitting BP measurements taken in repeated visits are these treatments. In this review, we present diagnostic fea- needed to confirm diagnosis of hypertension. Those should be tures of these conditions and discuss their management in ideally taken in a relaxed environment, with well-maintained particular, the endovascular techniques used by interven- equipment and with a rest period of at least 5 minutes before tional radiology to treat these conditions.