5.01.540 Miscellaneous Oncology Drugs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Phase I Trial of Tamoxifen with Ribociclib (LEE011) in Adult Patients with Advanced ER+ (HER2 Negative) Breast Cancer
The TEEL Study: A Phase I Trial of Tamoxifen with Ribociclib (LEE011) in Adult Patients with Advanced ER+ (HER2 Negative) Breast Cancer NCT02586675 Version 12.0 September 14, 2016 TEEL Protocol- Tamoxifen +Ribociclib Page 1 TITLE PAGE The TEEL Study: A Phase I trial of Tamoxifen with Ribociclib (LEE011) in adult patients with advanced ER+ (HER2 negative) breast cancer. Protocol: MCC 18332 Chesapeake IRB Pro00015228 Principal Investigator: Co-Investigators: Statistician: Experimental Therapeutics Program H. Lee Moffitt Cancer Center 12902 Magnolia Drive Tampa, FL 33612 & Comprehensive Breast Program Moffitt McKinley Outpatient Center 10920 N. McKinley Dr. Tampa, FL 33612 Study Site Contact: Protocol Version 12 Date: September 14, 2016 TEEL Protocol- Tamoxifen +Ribociclib Page 2 TITLE PAGE .............................................................................................................................................. 1 SYNOPSIS ................................................................................................................................................... 5 Patient Population ................................................................................................................................. 5 Type of Study ......................................................................................................................................... 5 Prior Therapy......................................................................................................................................... 5 -

PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects
International Journal of Molecular Sciences Review PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects Rosalin Mishra , Hima Patel, Samar Alanazi , Mary Kate Kilroy and Joan T. Garrett * Department of Pharmaceutical Sciences, College of Pharmacy, University of Cincinnati, Cincinnati, OH 45267-0514, USA; [email protected] (R.M.); [email protected] (H.P.); [email protected] (S.A.); [email protected] (M.K.K.) * Correspondence: [email protected]; Tel.: +1-513-558-0741; Fax: +1-513-558-4372 Abstract: The phospatidylinositol-3 kinase (PI3K) pathway is a crucial intracellular signaling pathway which is mutated or amplified in a wide variety of cancers including breast, gastric, ovarian, colorectal, prostate, glioblastoma and endometrial cancers. PI3K signaling plays an important role in cancer cell survival, angiogenesis and metastasis, making it a promising therapeutic target. There are several ongoing and completed clinical trials involving PI3K inhibitors (pan, isoform-specific and dual PI3K/mTOR) with the goal to find efficient PI3K inhibitors that could overcome resistance to current therapies. This review focuses on the current landscape of various PI3K inhibitors either as monotherapy or in combination therapies and the treatment outcomes involved in various phases of clinical trials in different cancer types. There is a discussion of the drug-related toxicities, challenges associated with these PI3K inhibitors and the adverse events leading to treatment failure. In addition, novel PI3K drugs that have potential to be translated in the clinic are highlighted. Keywords: cancer; PIK3CA; resistance; PI3K inhibitors Citation: Mishra, R.; Patel, H.; Alanazi, S.; Kilroy, M.K.; Garrett, J.T. -

Investigator Initiated Study IRB-29839 an Open-Label Pilot Study To
Investigator Initiated Study IRB-29839 An open-label pilot study to evaluate the efficacy and safety of a combination treatment of Sonidegib and BKM120 for the treatment of advanced basal cell carcinomas Version 05 September 2016 NCT02303041 DATE: 12Dec2018 1 Coordinating Center Stanford Cancer Center 875 Blake Wilbur Drive Stanford, CA 94305 And 450 Broadway, MC 5334 Redwood City, CA 94603 Protocol Director and Principal Investigator Anne Lynn S Chang, MD, Director of Dermatological Clinical Trials 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Co-Investigator Anthony Oro, MD PhD 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Biostatistician Shufeng Li, MS 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] Study Coordinator Ann Moffat 450 Broadway St, MC 5334 Redwood City, CA 94603 [email protected] 2 Table of Contents 1 Background ................................................................. 7 1.1 Disease Background ..................................................... 7 1.2 Hedgehog Pathway and mechanism of action ............................... 7 1.3 PI3K Pathway and mechanism of action ................................... 9 1.4 Sonidegib Compound Information ............ Error! Bookmark not defined. 1.4.1 Preclinical Studies for Sonidegib ....................................................................11 1.4.2 Muscular system...............................................................................................13 1.4.3 Skeletal system ................................................................................................13 -

PD-1/PD-L1 Targeting in Breast Cancer: the First Clinical Evidences Are Emerging
Supplementary Materials: PD-1/PD-L1 Targeting in Breast Cancer: the First Clinical Evidences are Emerging. A Literature Review Table S1. Interventional studies with anti PD-1 or PDL-1 agents recruiting. Non-intraveinous administrations are indicated between brackets. Estimated Estimated Study Ph. Anti-PD(L)-1 Single (S) or Combination Study title NCT Conditions or disease Enrollement Sponsor Completion Date (n=) Inflammatory and metastatic breast cancer (IBC) 1 Pembrolizumab S MK-3475 for Metastatic Inflammatory Breast Cancer (MIBC) 02411656 35 June 2020 MD Anderson or mTNBC Entinostat, Nivolumab, and Ipilimumab in treating patients Breast carcinoma: HER2 negative, Ipilimumab with solid tumors that are metastatic or cannot be removed by Invasive BC, Metastatic BC 1 Entinostat [PO] 02453620 45 December 2019 NCI Nivolumab surgery or locally advanced or metastatic HER2-NegativeBreast BC stage III, IIIA, IIIB, IIIC, IV Cancer Unresectable solid neoplasm Adjuvant PVX-410 Vaccine and Durvalumab in stage II/III TNBC stage II, III; HLA-A2 positive by Massachusetts 1 Durvalumab PVX-410 [IM] 02826434 20 December 2022 Triple Negative Breast Cancer deoxyribonucleic acid (DNA) sequence analysis General Hospit A Study of changes in PD-L1 expression during preoperative Pembrolizumab 1 Nab-paclitaxel treatment with Nab-Paclitaxel and pembrolizumab in Hormone 02999477 HR positive breast cancer 50 January 2023 Dana-Farber Receptor (HR) Positive BC Cisplatine PD1 Antibody + GP as first line treatment for triple negative Fudan 1 JS001 (anti PD1) Gemcitabine -

A Study of Abemaciclib (LY2835219)
A Study of Abemaciclib (LY2835219) in Combination With Temozolomide and Irinotecan and Abemaciclib in Combination With Temozolomide in Children and Young Adult Participants With Solid Tumors Status: Not yet recruiting Eligibility Criteria Sex: All Age: up to 18 Years old This study is NOT accepting healthy volunteers Inclusion Criteria: • Body weight ≥10 kilograms and body surface area (BSA) ≥0.5 meters squared. • Participants with any relapsed/refractory malignant solid tumor (excluding lymphoma), including central nervous system tumors, that have progressed on standard therapies and, in the judgment of the investigator, are appropriate candidates for the experimental therapy combination in the study part that is currently enrolling. • Participants must have at least one measurable (per Response Criteria in Solid Tumors [RECIST v1.1; [Eisenhauer et al. 2009] or Response Assessment in Neuro-Oncology (RANO) for central nervous system (CNS) tumors [Wen et al. 2010]) or evaluable lesion. • Participants must have had histologic verification of malignancy at original diagnosis or relapse, except: • Participants with extra-cranial germ-cell tumors who have elevations of serum tumor markers including alpha-fetoprotein or beta- human chorionic gonadotropin (HCG). • Participants with intrinsic brain stem tumors or participants with CNS-germ cell tumors and elevations of CSF or serum tumor markers including alpha-fetoprotein or beta-HCG. • A Lansky score ≥50 for participants ≤16 years of age or Karnofsky score ≥50 for participants >16 years of age. • Participants must have discontinued all previous treatments for cancer or investigational agents and must have recovered from the acute effects to Grade ≤1 at the time of enrollment. -

PARP Inhibitors Sensitize Ewing Sarcoma Cells to Temozolomide
Published OnlineFirst October 5, 2015; DOI: 10.1158/1535-7163.MCT-15-0587 Cancer Biology and Signal Transduction Molecular Cancer Therapeutics PARP Inhibitors Sensitize Ewing Sarcoma Cells to Temozolomide-Induced Apoptosis via the Mitochondrial Pathway Florian Engert1, Cornelius Schneider1, Lilly Magdalena Weib1,2,3, Marie Probst1,and Simone Fulda1,2,3 Abstract Ewing sarcoma has recently been reported to be sensitive to that subsequent to DNA damage-imposed checkpoint activa- poly(ADP)-ribose polymerase (PARP) inhibitors. Searching for tion and G2 cell-cycle arrest, olaparib/TMZ cotreatment causes synergistic drug combinations, we tested several PARP inhibi- downregulation of the antiapoptotic protein MCL-1, followed by tors (talazoparib, niraparib, olaparib, veliparib) together with activation of the proapoptotic proteins BAX and BAK, mitochon- chemotherapeutics. Here, we report that PARP inhibitors syner- drial outer membrane permeabilization (MOMP), activation of gize with temozolomide (TMZ) or SN-38 to induce apoptosis caspases, and caspase-dependent cell death. Overexpression of a and also somewhat enhance the cytotoxicity of doxorubicin, nondegradable MCL-1 mutant or BCL-2, knockdown of NOXA or etoposide, or ifosfamide, whereas actinomycin D and vincris- BAX and BAK, or the caspase inhibitor N-benzyloxycarbonyl-Val- tine show little synergism. Furthermore, triple therapy of ola- Ala-Asp-fluoromethylketone (zVAD.fmk) all significantly reduce parib, TMZ, and SN-38 is significantly more effective compared olaparib/TMZ-mediated apoptosis. These findings emphasize with double or monotherapy. Mechanistic studies revealed that the role of PARP inhibitors for chemosensitization of Ewing the mitochondrial pathway of apoptosis plays a critical role in sarcoma with important implications for further (pre)clinical mediating the synergy of PARP inhibition and TMZ. -

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

R&D Briefing 76
The Impact of Recent Regulatory Developments on the Mexican Therapeutic Landscape Access to innovative medicines is key to El acceso a medicamentos innovadores es clave para improving overall population health, reducing mejorar la salud de toda la población, para reducir los hospitalisation time and decreasing morbidity and tiempos de hospitalización, la morbilidad y la mortalidad mortality. An efficient regulatory process can be de un país. Un proceso regulatorio eficiente tiene un reflected in measurable positive health impacts; impacto positivo medible en la salud, y por el contrario, conversely, activities that slow or impede acciones que retrasan o impiden la eficiencia regulatoria y regulatory efficiency and predictability can be su predictibilidad pueden ser perjudiciales. La parálisis detrimental. Recent developments in the Mexican reciente del Sistema regulatorio mexicano respecto a la regulatory system for the assessments of evaluación de nuevos medicamentos innovadores innovative new products have had a negative conlleva un impacto negativo en la salud de la población impact on Mexican public health. mexicana. This Briefing addresses the impact of suspending Este informe analiza el impacto de la suspensión de las the activities of the New Molecules Committee actividades del Comité de Moléculas Nuevas (NMC, por (NMC) on the Mexican therapeutic landscape. sus siglas en inglés) sobre el horizonte terapéutico de First, we compared the way that “new medicines” México. En primer lugar, comparamos la definición de are defined within the context of the Mexican nuevos medicamentos según el contexto regulatorio regulatory system, with definitions used by mexicano con las definiciones adoptadas por otras comparable regulators and health organisations. agencias reguladoras u organizaciones de salud del We have also investigated the extent to which mundo. -

Cancer Drug Pharmacology Table
CANCER DRUG PHARMACOLOGY TABLE Cytotoxic Chemotherapy Drugs are classified according to the BC Cancer Drug Manual Monographs, unless otherwise specified (see asterisks). Subclassifications are in brackets where applicable. Alkylating Agents have reactive groups (usually alkyl) that attach to Antimetabolites are structural analogues of naturally occurring molecules DNA or RNA, leading to interruption in synthesis of DNA, RNA, or required for DNA and RNA synthesis. When substituted for the natural body proteins. substances, they disrupt DNA and RNA synthesis. bendamustine (nitrogen mustard) azacitidine (pyrimidine analogue) busulfan (alkyl sulfonate) capecitabine (pyrimidine analogue) carboplatin (platinum) cladribine (adenosine analogue) carmustine (nitrosurea) cytarabine (pyrimidine analogue) chlorambucil (nitrogen mustard) fludarabine (purine analogue) cisplatin (platinum) fluorouracil (pyrimidine analogue) cyclophosphamide (nitrogen mustard) gemcitabine (pyrimidine analogue) dacarbazine (triazine) mercaptopurine (purine analogue) estramustine (nitrogen mustard with 17-beta-estradiol) methotrexate (folate analogue) hydroxyurea pralatrexate (folate analogue) ifosfamide (nitrogen mustard) pemetrexed (folate analogue) lomustine (nitrosurea) pentostatin (purine analogue) mechlorethamine (nitrogen mustard) raltitrexed (folate analogue) melphalan (nitrogen mustard) thioguanine (purine analogue) oxaliplatin (platinum) trifluridine-tipiracil (pyrimidine analogue/thymidine phosphorylase procarbazine (triazine) inhibitor) -

Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non–Small Cell Lung Cancer, and Other Solid Tumors
Published OnlineFirst May 23, 2016; DOI: 10.1158/2159-8290.CD-16-0095 RESEARCH ARTICLE Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non–Small Cell Lung Cancer, and Other Solid Tumors Amita Patnaik1, Lee S. Rosen2, Sara M. Tolaney3, Anthony W. Tolcher1, Jonathan W. Goldman2, Leena Gandhi3, Kyriakos P. Papadopoulos1, Muralidhar Beeram1, Drew W. Rasco1, John F. Hilton3, Aejaz Nasir4, Richard P. Beckmann4, Andrew E. Schade4, Angie D. Fulford4, Tuan S. Nguyen4, Ricardo Martinez4, Palaniappan Kulanthaivel4, Lily Q. Li4, Martin Frenzel4, Damien M. Cronier4, Edward M. Chan4, Keith T. Flaherty5, Patrick Y. Wen3, and Geoffrey I. Shapiro3 ABSTRACT We evaluated the safety, pharmacokinetic profile, pharmacodynamic effects, and antitumor activity of abemaciclib, an orally bioavailable inhibitor of cyclin-dependent kinases (CDK) 4 and 6, in a multicenter study including phase I dose escalation followed by tumor- specific cohorts for breast cancer, non–small cell lung cancer (NSCLC), glioblastoma, melanoma, and colorectal cancer. A total of 225 patients were enrolled: 33 in dose escalation and 192 in tumor-specific cohorts. Dose-limiting toxicity was grade 3 fatigue. The maximum tolerated dose was 200 mg every 12 hours. The most common possibly related treatment-emergent adverse events involved fatigue and the gastrointestinal, renal, or hematopoietic systems. Plasma concentrations increased with dose, and pharmacodynamic effects were observed in proliferating keratinocytes and tumors. Radiographic responses were achieved in previously treated patients with breast cancer, NSCLC, and melanoma. For hormone receptor–positive breast cancer, the overall response rate was 31%; moreover, 61% of patients achieved either response or stable disease lasting ≥6 months. -
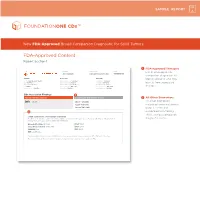
FDA-Approved Content Report Section 1
SAMPLE REPORT New FDA-Approved Broad Companion Diagnostic for Solid Tumors FDA-Approved Content Report Section 1 1 FDA-Approved Therapies PATIENT TUMOR TYPE TRF# List of FDA-approved Jane Sample Lung adenocarcinoma TRFXXXXXX companion diagnostics to PATIENT PHYSICIAN SPECIMEN identify patients who may DISEASE Lung adenocarcinoma ORDERING PHYSICIAN Not Given SPECIMEN SITE Not Given NAME Not Given MEDICAL FACILITY Not Given SPECIMEN ID Not Given benefi t from associated DATE OF BIRTH Not Given ADDITIONAL RECIPIENT Not Given SPECIMEN TYPE Not Given SEX Female MEDICAL FACILITY ID Not Given DATE OF COLLECTION Not Given therapies MEDICAL RECORD # Not Given PATHOLOGIST Not Given SPECIMEN RECEIVED Not Given CDx Associated Findings 1 GENOMIC FINDINGS DETECTED FDA-APPROVED THERAPEUTIC OPTIONS 2 All Other Biomarkers EGFR L858R Gilotrif® (Afatinib) All other biomarkers, Iressa® (Gefitinib) including tumor mutational Tarceva® (Erlotinib) burden (TMB) and 2 microsatellite instability (MSI), without companion OTHER ALTERATIONS & BIOMARKERS IDENTIFIED Results reported in this section are not prescriptive or conclusive for labeled use of any specific therapeutic product. See diagnostic claims professional services section for additional information. Microsatellite Status MS-Stable PTCH1 T416S Tumor Mutation Burden 11 Muts/Mb RBM10 Q494* CDKN2A/B loss TP53 R267P EGFR amplification § Refer to appendix for limitation statements related to detection of any copy number alterations, gene rearrangements, MSI or TMB result in this section. Please refer to appendix