Prescribing Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1: Clinical Pharmacokinetics 1
1: CLINICAL PHARMACOKINETICS 1 General overview: clinical pharmacokinetics, 2 Pharmacokinetics, 4 Drug clearance (CL), 6 Volume of distribution (Vd), 8 The half-life (t½), 10 Oral availability (F), 12 Protein binding (PB), 14 pH and pharmacokinetics, 16 1 Clinical pharmacokinetics General overview General overview: clinical pharmacokinetics 1 The ultimate aim of drug therapy is to achieve effi cacy without toxicity. This involves achieving a plasma concentration (Cp) within the ‘therapeutic window’, i.e. above the min- imal effective concentration (MEC), but below the minimal toxic concentration (MTC). Clinical pharmacokinetics is about all the factors that determine variability in the Cp and its time-course. The various factors are dealt with in subsequent chapters. Ideal therapeutics: effi cacy without toxicity Minimum Toxic Concentration (MTC) Ideal dosing Minimum Effective Concentration (MEC) Drug concentration Time The graph shows a continuous IV infusion at steady state, where the dose-rate is exactly appropriate for the patient’s clearance (CL). Inappropriate dosing Dosing too high in relation to the patient’s CL – toxicity likely Minimum Toxic Concentration (MTC) Minimum Effective Concentration (MEC) Dosing too low in relation to the Drug concentration patient’s CL – drug may be ineffective Time Some reasons for variation in CL Low CL High CL Normal variation Normal variation Renal impairment Increased renal blood fl ow Genetic poor metabolism Genetic hypermetabolism Liver impairment Enzyme induction Enzyme inhibition Old age/neonate 2 General overview Clinical Pharmacokinetics Pharmacokinetic factors determining ideal therapeutics If immediate effect is needed, a loading dose (LD) must be given to achieve a desired 1 concentration. The LD is determined by the volume of distribution (Vd). -

STATUS EPILEPTICUS in ADULTS (Convulsive Seizures in Patients Aged > 16 Years Old) Link Consultant: Dr Hannah Cock
STATUS EPILEPTICUS IN ADULTS (Convulsive Seizures in patients aged > 16 years old) Link consultant: Dr Hannah Cock Status epilepticus (SE) is defined as continuous seizure activity which has failed to self- terminate leading to a risk of neurological damage. The risks are highest with generalised tonic/clonic (convulsive) seizures. Convulsive SE may present as either a run of discreet generalised tonic/clonic seizures without full recovery in between (ie without regaining consciousness), or continuous generalised tonic/clonic seizure activity. Most convulsive seizures terminate spontaneously within 3 minutes, and do NOT need emergency treatment. Convulsive seizures lasting longer than 5 minutes, or recurring without recovery should be managed as Convulsive SE, unless the patient is known to habitually have longer seizures with self-termination (eg information from relatives, friends, or the patient’s epilepsy card or diary). The mortality and morbidity of generalised status epilepticus is high, and it is important to control fits as soon as possible, to use adequate doses of 1st and 2nd line agents, but not to over-treat patients in whom seizures have terminated but are slow to recover. GENERAL MANAGEMENT 1st stage (0-10mins). Protect the patient e.g. padded bed rails. Do not restrain. Administer oxygen. During an inter-ictal period insert an airway and then administer oxygen. Do not attempt to insert anything in the patient’s mouth during a seizure, even if the tongue is injured. Place the patient in a semi-prone position with the head down to prevent aspiration. Establish iv access. Note the time. 2nd Stage (0-30mins). Institute regular monitoring (temperature, cardiac, respiration, BP). -

Restless Legs Syndrome in Patients with Epilepsy Under Levetiracetam Monotherapy
Original Article / Özgün Makale DODO I: 10.4274/ I: 10.4274/jtsm.xxxjtsm.69188 Journal of Turkish Sleep Medicine 2018;5:12-6 Restless Legs Syndrome in Patients with Epilepsy Under Levetiracetam Monotherapy Levetirasetam Monoterapisi Altında Epilepsi Hastalarında Huzursuz Bacak Sendromu Gülnihal Kutlu, Fatma Genç*, Yasemin Ünal, Dilek Aslan Öztürk, Abidin Erdal*, Yasemin Biçer Gömceli* Muğla Sıtkı Koçman University Faculty of Medicine, Department of Neurology, Muğla, Turkey *University of Health Sciences, Antalya Training and Research Hospital, Clinic of Neurology, Antalya, Turkey Abstract Öz Objective: Restless Legs syndrome (RLS) is a frequent neurological Amaç: Huzursuz Bacak sendromu (HBS) sık görülen bir nörolojik disease. Levetiracetam (LEV) is an effective and broad-spectrum hastalıktır. Levetirasetam (LEV) etkili ve geniş spektrumlu bir antiepileptik anticonvulsant drug. The aim of this study is to investigate the frequency ilaçtır. Bu çalışmanın amacı epilepsi tanısı ile LEV monoterapisi alan of RLS in patients diagnosed with epilepsy who took LEV monotherapy. hastalarda HBS sıklığını araştırmaktır. Materials and Methods: Two neurologists were reviewed the files of Gereç ve Yöntem: Epilepsi polikliniğinde takip edilen 1680 hastanın 1680 patients, who were followed in epilepsy outpatient clinic. One dosyası iki nörolog tarafından gözden geçirildi. En az 6 aydır LEV hundred seven patients under LEV monotherapy for at least six months monoterapisi alan 107 hasta ve 120 sağlıklı kontrol çalışmaya alındı. and 120 healthy controls were included in the study. The criteria for the International Restless Legs Syndrome Study Group were taken into HBS değerlendirmesi için Uluslararası Huzursuz Bacak Sendromu Çalışma consideration for the assessment of RLS. Grubu’nun kriterleri göz önüne alındı. Results: The mean age of patient group was 38.26±17.39 years, while Bulgular: Sağlıklı kontrollerin ortalama yaşı 39,17±16,12 yıl iken, the mean age of healthy controls was 39.17±16.12 years. -

Attachment: Product Information Brivaracetam
Attachment 1: Product information for AusPAR AusPAR Briviact UCB Australia Pty Ltd PM- 2015-01568-1-1 Final 7 March 2017. This Product Information was approved at the time this AusPAR was published. PRODUCT INFORMATION BRIVIACT (brivaracetam) Solution for Injection NAME OF THE MEDICINE Non-proprietary name: Brivaracetam Chemical name: (2S)-2-[(4R)-2-oxo-4-propyltetrahydro-1H-pyrrol-1-yl]butanamide Chemical structure: Molecular formula: C11H20N2O2 MW: 212.29 CAS number: [357336-20-0] DESCRIPTION The active ingredient brivaracetam is a white to off-white crystalline powder. It is very soluble in water, buffer (pH 1.2, 4.5 and 7.4), ethanol, methanol, and glacial acetic acid. It is freely soluble in acetonitrile and acetone and soluble in toluene. It is very slightly soluble in n-hexane. BRIVIACT solution for injection contains the following excipients: sodium acetate trihydrate, glacial acetic acid, sodium chloride, water for injections. PHARMACOLOGY Mechanism of action Brivaracetam displays a high and selective affinity for synaptic vesicle protein 2A (SV2A) in the brain. Binding to SV2A is considered to be the primary mechanism for brivaracetam anticonvulsant activity, however, the precise mechanism by which brivaracetam exerts is anticonvulsant activity has not been fully elucidated. Effects on QT interval The effect of brivaracetam on QTc prolongation was evaluated in a randomized, double-blind, positive-controlled (moxifloxacin 400 mg)- and placebo-controlled parallel group study of brivaracetam (150 mg/day and 800 mg/day in two daily intakes) in 184 healthy subjects. There was no evidence that brivaracetam prolongs the QT interval. Seizure frequency Briviact Solution for injection PI g2016-263 Page 1 Attachment 1: Product information for AusPAR AusPAR Briviact UCB Australia Pty Ltd PM- 2015-01568-1-1 Final 7 March 2017. -

Epilepsy & Behavior
Epilepsy & Behavior 80 (2018) 365–369 Contents lists available at ScienceDirect Epilepsy & Behavior journal homepage: www.elsevier.com/locate/yebeh Brief Communication Eslicarbazepine acetate as a replacement for levetiracetam in people with epilepsy developing behavioral adverse events Virupakshi Jalihal a, Rohit Shankar b,c,⁎, William Henley c, Mary Parrett d, Phil Tittensor e, Brendan N. McLean d, Ammad Ahmed f, Josemir W. Sander g,h,i a Ramaiah Medical College and Hospitals, Bengaluru, Karnataka 560054, India b Cornwall Partnership NHS Foundation Trust, Threemilestone Industrial Estate, Truro TR4 9LD, UK c Exeter Medical School, Knowledge Spa, Royal Cornwall Hospital, Truro, Cornwall TR1 3HD, UK d Royal Cornwall Hospital, Truro, Cornwall TR1 3LJ, UK e Royal Wolverhampton NHS Trust, UK f Bial Pharma Ltd., Admiral House, Windsor SL4 3BL, UK g NIHR University College London Hospitals Biomedical Research Centre, UCL Institute of Neurology, Queen Square, London WC1N 3BG, UK h Chalfont Centre for Epilepsy, Chalfont St Peter, Buckinghamshire SL9 0RJ, UK i Stichting Epilepsie Instellingen Nederland (SEIN), Achterweg 5, 2103 SW Heemstede, Netherlands article info abstract Article history: Background: Psychiatric and behavioral side effects (PBSEs) are a major cause of antiepileptic drug (AED) Received 13 November 2017 withdrawal. Levetiracetam (LEV) is a recognized first-line AED with good seizure outcomes but recognized Revised 16 January 2018 with PBSEs. Eslicarbazepine (ESL) is considered to function similarly to an active metabolite of the commonly Accepted 17 January 2018 used carbamazepine (CBZ). Carbamazepine is used as psychotropic medication to assist in various psychiatric Available online 5 February 2018 illnesses such as mood disorders, aggression, and anxiety. -

Mechanisms of Action of Antiepileptic Drugs
Review Mechanisms of action of antiepileptic drugs Epilepsy affects up to 1% of the general population and causes substantial disability. The management of seizures in patients with epilepsy relies heavily on antiepileptic drugs (AEDs). Phenobarbital, phenytoin, carbamazepine and valproic acid have been the primary medications used to treat epilepsy for several decades. Since 1993 several AEDs have been approved by the US FDA for use in epilepsy. The choice of the AED is based primarily on the seizure type, spectrum of clinical activity, side effect profile and patient characteristics such as age, comorbidities and concurrent medical treatments. Those AEDs with broad- spectrum activity are often found to exert an action at more than one molecular target. This article will review the proposed mechanisms of action of marketed AEDs in the US and discuss the future of AEDs in development. 1 KEYWORDS: AEDs anticonvulsant drugs antiepileptic drugs epilepsy Aaron M Cook mechanism of action seizures & Meriem K Bensalem-Owen† The therapeutic armamentarium for the treat- patients with refractory seizures. The aim of this 1UK HealthCare, 800 Rose St. H-109, ment of seizures has broadened significantly article is to discuss the past, present and future of Lexington, KY 40536-0293, USA †Author for correspondence: over the past decade [1]. Many of the newer AED pharmacology and mechanisms of action. College of Medicine, Department of anti epileptic drugs (AEDs) have clinical advan- Neurology, University of Kentucky, 800 Rose Street, Room L-455, tages over older, so-called ‘first-generation’ First-generation AEDs Lexington, KY 40536, USA AEDs in that they are more predictable in their Broadly, the mechanisms of action of AEDs can Tel.: +1 859 323 0229 Fax: +1 859 323 5943 dose–response profile and typically are associ- be categorized by their effects on the neuronal [email protected] ated with less drug–drug interactions. -

Chapter 25 Mechanisms of Action of Antiepileptic Drugs
Chapter 25 Mechanisms of action of antiepileptic drugs GRAEME J. SILLS Department of Molecular and Clinical Pharmacology, University of Liverpool _________________________________________________________________________ Introduction The serendipitous discovery of the anticonvulsant properties of phenobarbital in 1912 marked the foundation of the modern pharmacotherapy of epilepsy. The subsequent 70 years saw the introduction of phenytoin, ethosuximide, carbamazepine, sodium valproate and a range of benzodiazepines. Collectively, these compounds have come to be regarded as the ‘established’ antiepileptic drugs (AEDs). A concerted period of development of drugs for epilepsy throughout the 1980s and 1990s has resulted (to date) in 16 new agents being licensed as add-on treatment for difficult-to-control adult and/or paediatric epilepsy, with some becoming available as monotherapy for newly diagnosed patients. Together, these have become known as the ‘modern’ AEDs. Throughout this period of unprecedented drug development, there have also been considerable advances in our understanding of how antiepileptic agents exert their effects at the cellular level. AEDs are neither preventive nor curative and are employed solely as a means of controlling symptoms (i.e. suppression of seizures). Recurrent seizure activity is the manifestation of an intermittent and excessive hyperexcitability of the nervous system and, while the pharmacological minutiae of currently marketed AEDs remain to be completely unravelled, these agents essentially redress the balance between neuronal excitation and inhibition. Three major classes of mechanism are recognised: modulation of voltage-gated ion channels; enhancement of gamma-aminobutyric acid (GABA)-mediated inhibitory neurotransmission; and attenuation of glutamate-mediated excitatory neurotransmission. The principal pharmacological targets of currently available AEDs are highlighted in Table 1 and discussed further below. -

Mode of Seizure Inhibition by Sodium Channel Blockers, an SV2A Ligand
Epilepsy Research 154 (2019) 42–49 Contents lists available at ScienceDirect Epilepsy Research journal homepage: www.elsevier.com/locate/epilepsyres Mode of seizure inhibition by sodium channel blockers, an SV2A ligand, and T an AMPA receptor antagonist in a rat amygdala kindling model ⁎ Ting Wua, Katsutoshi Idoa, Makoto Ohgoha, Takahisa Hanadab, a Neurology Tsukuba Research Department, Discovery, Medicine Creation, Neurology Business Group, Eisai Co., Ltd. Japan b Clinical Science Department, Medical Division, Eisai Co., Ltd. Nishigokencho 13-1, Shinjuku-ku, Tokyo 162-0812, Japan ARTICLE INFO ABSTRACT Keywords: Purpose: A number of antiepileptic drugs (AEDs) with a variety of modes of action, are effective in treating focal AMPA receptor antagonist seizures. Several AEDs, such as perampanel (PER), levetiracetam (LEV), lacosamide (LCM), lamotrigine (LTG), Antiepileptic drug and carbamazepine (CBZ), have been shown to elevate the seizure threshold in kindling models. These AEDs are Mode of seizure inhibition clinically effective, but differences exist in the anti-seizure profiles of drugs with similar modes ofaction. Focal seizure Therefore, we hypothesized that there are differences in how these AEDs affect seizures. Here, we evaluated the Perampanel effects of AEDs on various seizure parameters in a rat amygdala kindling model upon stimulation attheafter- Synaptic transmission discharge threshold (ADT) and at three-times the ADT (3xADT) to characterize the differences in the effects of these AEDs. Methods: PER, LEV, LCM, LTG, CBZ, or vehicle was administered intraperitoneally to fully kindled rats. Changes in Racine seizure score, after-discharge duration (ADD), and latency to Racine score 4 generalized seizure (S4L) were measured to assess differences in the modes of seizure inhibition among the AEDs. -

CPIC Guideline for Pharmacogenetics-Guided Warfarin Dosing – Supplement V2.0 1 Table of Contents Guideline Updates
Supplement to: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for Pharmacogenetics-guided Warfarin Dosing: 2016 Update Julie A. Johnson1, Kelly Caudle2, Li Gong3, Michelle Whirl-Carrillo3, C. Michael Stein4, Stuart A. Scott5, Ming Ta Michael Lee6 , Brian F. Gage7, Stephen E. Kimmel8,9, Minoli A. Perera10, Jeffrey L. Anderson11, Munir Pirmohamed12, Teri E. Klein3, Nita A. Limdi13, Larisa H. Cavallari1, Mia Wadelius14 1Department of Pharmacotherapy and Translational Research, College of Pharmacy, and Center for Pharmacogenomics, University of Florida, Gainesville, Florida, USA 2Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, TN 3Department of Genetics, Stanford University, Stanford, California, USA 4Division of Clinical Pharmacology Vanderbilt Medical School, Nashville, TN, USA 5Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA 6Laboratory for International Alliance on Genomic Research, RIKEN Center for Integrative Medical Sciences, Yokohama, Japan; National Center for Genome Medicine; Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan; Genomic Medicine Institute Geisinger Health system, Danville, PA 7Department of Internal Medicine, Washington University in St. Louis, St. Louis, Missouri 8Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA 9Department of Medicine and Department of Biostatistics and Epidemiology, University of Pennsylvania -

Efavirenz) Capsules and Tablets 3 Rx Only
1 SUSTIVA® 2 (efavirenz) capsules and tablets 3 Rx only 4 DESCRIPTION 5 SUSTIVA® (efavirenz) is a human immunodeficiency virus type 1 (HIV-1) specific, non- 6 nucleoside, reverse transcriptase inhibitor (NNRTI). 7 Capsules: SUSTIVA is available as capsules for oral administration containing either 8 50 mg, 100 mg, or 200 mg of efavirenz and the following inactive ingredients: lactose 9 monohydrate, magnesium stearate, sodium lauryl sulfate, and sodium starch glycolate. 10 The capsule shell contains the following inactive ingredients and dyes: gelatin, sodium 11 lauryl sulfate, titanium dioxide, and/or yellow iron oxide. The capsule shells may also 12 contain silicon dioxide. The capsules are printed with ink containing carmine 40 blue, 13 FD&C Blue No. 2, and titanium dioxide. 14 Tablets: SUSTIVA is available as film-coated tablets for oral administration containing 15 600 mg of efavirenz and the following inactive ingredients: croscarmellose sodium, 16 hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline 17 cellulose, and sodium lauryl sulfate. The film coating contains Opadry® Yellow and 18 Opadry® Clear. The tablets are polished with carnauba wax and printed with purple ink, 19 Opacode® WB. 20 Efavirenz is chemically described as (S)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4- 21 (trifluoromethyl)-2H-3,1-benzoxazin-2-one. 22 Its empirical formula is C14H9ClF3NO2 and its structural formula is: 1 of 45 Approved v2.0 F C 3 Cl O NO 23 H 24 Efavirenz is a white to slightly pink crystalline powder with a molecular mass of 315.68. 25 It is practically insoluble in water (<10 µg/mL). -
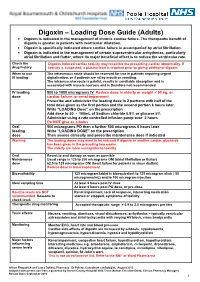
Digoxin – Loading Dose Guide (Adults) Digoxin Is Indicated in the Management of Chronic Cardiac Failure
Digoxin – Loading Dose Guide (Adults) Digoxin is indicated in the management of chronic cardiac failure. The therapeutic benefit of digoxin is greater in patients with ventricular dilatation. Digoxin is specifically indicated where cardiac failure is accompanied by atrial fibrillation. Digoxin is indicated in the management of certain supraventricular arrhythmias, particularly atrial fibrillation and flutter, where its major beneficial effect is to reduce the ventricular rate. Check the Digoxin-induced cardiac toxicity may resemble the presenting cardiac abnormality. If drug history toxicity is suspected, a plasma level is required prior to giving additional digoxin. When to use The intravenous route should be reserved for use in patients requiring urgent IV loading digitalisation, or if patients are nil by mouth or vomiting. The intramuscular route is painful, results in unreliable absorption and is associated with muscle necrosis and is therefore not recommended. IV loading 500 to 1000 micrograms IV Reduce dose in elderly or weight < 50 kg, or dose cardiac failure, or renal impairment Prescribe and administer the loading dose in 2 portions with half of the total dose given as the first portion and the second portion 6 hours later. Write “LOADING Dose” on the prescription Add dose to 50 - 100mL of Sodium chloride 0.9% or glucose 5% Administer using a rate controlled infusion pump over 2 hours Do NOT give as a bolus Oral 500 micrograms PO then a further 500 micrograms 6 hours later loading Write “LOADING DOSE” on the prescription dose Then assess clinically and prescribe maintenance dose if indicated Warning The loading doses may need to be reduced if digoxin or another cardiac glycoside has been given in the preceding two weeks. -

Liste Over 2 Høringssvar -N03
Svar på Medicintilskudsnævnets høring over nævnets 2. forslag af 30. september 2013 til tilskudsstatus for lægemidler mod epilepsi Vi har modtaget høringssvar fra følgende: • Danmarks Apotekerforening • Dansk Epilepsiforening • Dansk Epilepsi Selskab • Dansk Neuropædiatrisk Selskab • Desitin Pharma A/S • Eisai AB • ERA Medical ApS Medicintilskudsnævnet, den 22. november 2013. Danmarks Apotekerforening Kanonbådsvej 10 · Postboks 2181 · 1017 København K Telefon 33 76 76 00 · Fax 33 76 76 99 [email protected] · www.apotekerforeningen.dk Til Medicintilskudsnævnet 29. oktober 2013 GHE/610/00005 Høring over Medicintilskudsnævnets 2. forslag til fremtidig tilskudsstatus for lægemidler mod epilepsi i ATC-gruppe N03, N05BA og N05CD Medicintilskudsnævnet har med meddelelse af 30. september 2013 udsendt 2. forslag til fremtidig tilskudsstatus for lægemidler mod epilepsi. Det fremgår af høringsskrivelsen, at udarbejdelsen af det 2. forslag er foranlediget af de høringssvar, der er indkommet efter 1. forslag. Apotekerforeningen finder det positivt, at Medicintilskudsnævnet har lyttet til hørings- parterne, og i sit 2. forslag til indstilling for nogle lægemidler nu anbefaler en mindre re- striktiv tilskudsstatus, end nævnet lagde op til i sit første forslag. Apotekerforeningen noterer sig dog, at Medicintilskudsnævnets 2. forslag til revurdering af tilskudsstatus for epilepsimidlerne stadig indeholder forslag om ændringer i tilskuds- status for mere end halvdelen af alle vurderede grupper. Foreningen undrer sig over, at Medicintilskudsnævnet for alle disse lægemidler anbefaler en mere restriktiv tilskudssta- tus, der i praksis vil gøre det vanskeligere for de berørte patienter at få beregnet tilskud, når nævnet mener, at forbrugsmønstret tyder på, at forbruget af lægemidlerne i dag er hensigtsmæssigt og rationelt. Medicintilskudsnævnets 2. forslag vil medføre, at lægemidlerne i over halvdelen af de revurderede grupper vil få tildelt klausuleret tilskud.