Somani Girdharilal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Main S.No./State S.No Name of the Instt./ Hospital No. of Candidates Approved for Training Under E.R.1991. Andhra Pradesh 1/1 Bh
main S.No./state Name of the Instt./ Hospital No. of candidates approved S.No for training under E.R.1991. Andhra Pradesh 1/1 Bharat Heavy Electricals Ltd. 14 General Hospital Ramachandrapuram Hyderabad - 502 032. 2/2 MNJ Institute of Oncology and 4 Regional Cancer Centre Red Hills, Hyderabad – 500 004, (Andhra Pradesh) 3/3 South Central Railway 4 Health Unit Chilkalguda Secunderabad – 25 4/4 Care Pharmacy 11 M/s Quality Care India Limited, Care Hospital, D.No. 06-03-248/2, Road No. 1, Banjara Hills Hyderabad Karnataka 5/1 National Institute of Mental Health and 6 Neuro Sciences (Deemed University) Post Bag No. 2900, Hosur Road Bangalore-560 029. (under MOH & F.W. Govt. of India) 6/2 5 National MineralDevelopment Corporation Limited (Govt. of India undertaking) 7/3 NET’s Navodaya Medical College 4 Hospital & Research Centre, P.B. No.26 Raichur – 584 103 8/4 Adichunchanagiri Hospital and Research Center (AH & RC) 6 main S.No./state Name of the Instt./ Hospital No. of candidates approved S.No for training under E.R.1991. Kerala 9/1 A mrita Institute of Medical Sciences 47 (inclu ding 10 and Research Centre, Amrita Lane, already appr oved by Elamakkara P.O.Cochin – 682 026 PCI) (Kerala) 10/2 K .V.M. Hospital, P.B.No.30, 4 Cherthala – 688 524, (Kerala) 11/3 Sree Uthradom Thirunal 9 Hospital, Pattom Thiruvananthapuram Distt. 12/4 Elite Mission Hospital Raise from3 to 5 Candidates Koorkencherry, Trichur Kerala. 13/5 Ebenezer Hospital 4 Kayamkulam. (Kerala) 14/6 Little Flower Hospital & Research 16 Centre Angamaly, Ernakulam (Kerala) 15/7 Edappal Hospital P.O. -

Little Flower Hospital
+91-8048372104 Little Flower Hospital https://www.indiamart.com/little-flower-hospital-trust/ Offering lab services, nursing services, bio medical services etc. About Us The hospital had a very humble beginning in 1936. Angarnaly was a tiny village with barely any facility to address the health needs, of the local community. It was with this in mind that the Archdiocese of Emakulam started this hospital. The hospital was named after St.Theresa of Lisieux who is the patron saint of the hospital (She is lovingly called 'The Little Flower’). Little Flower Hospital and Research Centre is a not for profit organization registered as a charitable trust. It is an 850 bedded multi speciality hospital. It also Imparts training in various fields of Medical, Paramedical and Nursing care. It is also a recognized research centre. The hospital is conveniently located with easy access by road, rail and air. Cochin International Airport is only 4 km away and the nearest railway station is only 1.5km away from the hospital. The district headquarters, Ernakularn is also near to the hospital. Angamaly is the meeting point of National Highway 47 and the Main Central Road. For more information, please visit https://www.indiamart.com/little-flower-hospital-trust/aboutus.html OTHER SERVICES P r o d u c t s & S e r v i c e s Cssd (Central Sterile Supplies Medical Records Department) Library Blood Bank P r o OTHER PRODUCTS: d u c t s & S e r v i c e s Pharmacy Lab Services Blood Bank Blood Bank F a c t s h e e t Year of Establishment : 1936 Nature of Business : Buyer-Individual CONTACT US Little Flower Hospital Contact Person: Prasad VK 7 7/560-568 M C Road Little Flower Hospital Building Angamaly Angamaly - 683572, Kerala, India +91-8048372104 https://www.indiamart.com/little-flower-hospital-trust/. -

St. Joseph's College for Women, Tirupur, Tamilnadu
==================================================================== Language in India www.languageinindia.com ISSN 1930-2940 Vol. 18:10 October 2018 India’s Higher Education Authority UGC Approved List of Journals Serial Number 49042 ==================================================================== St. Joseph’s College for Women, Tirupur, Tamilnadu R. Rajalakshmi, Editor Select Papers from the Conference Reading the Nation – The Global Perspective • Greetings from the Principal ... Rev. Sr. Dr. Kulandai Therese. A i • Editor's Preface ... R. Rajalakshmi, Assistant Professor and Head Department of English ii • Caste and Nation in Indian Society ... CH. Chandra Mouli & B. Sridhar Kumar 1-16 =============================================================================== Language in India www.languageinindia.com ISSN 1930-2940 18:10 October 2018 R. Rajalakshmi, Editor: Reading the Nation – The Global Perspective • Nationalism and the Postcolonial Literatures ... Dr. K. Prabha 17-21 • A Study of Men-Women Relationship in the Selected Novels of Toni Morrison ... G. Giriya, M.A., B.Ed., M.Phil., Ph.D. Research Scholar & Dr. M. Krishnaraj 22-27 • Historicism and Animalism – Elements of Convergence in George Orwell’s Animal Farm ... Ms. Veena SP 28-34 • Expatriate Immigrants’ Quandary in the Oeuvres of Bharati Mukherjee ... V. Jagadeeswari, Assistant Professor of English 35-41 • Post-Colonial Reflections in Peter Carey’s Journey of a Lifetime ... Meera S. Menon II B.A. English Language & Literature 42-45 • Retrieval of the Mythical and Dalit Imagination in Cho Dharman’s Koogai: The Owl ... R. Murugesan Ph.D. Research Scholar 46-50 • Racism in Nadine Gordimer’s The House Gun ... Mrs. M. Nathiya Assistant Professor 51-55 • Mysteries Around the Sanctum with Special Reference To The Man From Chinnamasta by Indira Goswami ... Mrs. T. Vanitha, M.A., M.Ed., M.Phil., Ph.D. -

Curiculum Vitae
CURICULUM VITAE NAME: Dr. DEEPA PAULOSE FRCS (Glasgow) DATE OF BIRTH: 14th November 1971 MARITAL STATUS: Married NATIONALITY: Indian E – MAIL ADDRESS [email protected] PRESENT EMPLOYMENT: Specialist Ophthalmologist American Mission Hospital Kingdom of Bahrain. from May 2012 EXPERTISE IN OPTHALMOLOGY PROCEDURES: o Trabeculectomy, o Phacoemulsification with foldable intraocular lens implantation, o Small incision cataract surgeries, o Extra capsular cataract surgeries, o Pterygium with conjunctival autograft, o Traumatic corneal tear, scleral tear, lid tear repairs o LASIK surgeries. PRESENT AND PREVIOUS EMPLOYMENT: 1. Specialist Ophthalmologist American Mission Hospital Manama, Kingdom of Bahrain. May 2012 till date Telephone: +973 1725 3447 2. Specialist Ophthalmologist KIMS Medical Center, Umm al Hassam, Manama Kingdom of Bahrain. March 2011 till Jan 2012 Telephone: +973 1782 2123 3. Consultant Ophthalmologist and Head of Glaucoma Unit Dr. Tony’s Superspeciality Eye Institute, Aluva, Kerala, India, March 2009 till Sept 2010. Telephone: +91 484 2623370 4. Junior Consultant and Camp Medical Officer at the Department of Ophthalmology, CBM OPHTHALMIC INSTITUTE, LITTLE FLOWER HOSPITAL, Angamaly July 2005 till March 2009. Telephone: +91 484 2452546 5. ICMR project Officer in charge of Glaucoma, at Department of Ophthalmology Little Flower Hospital, Angamaly August 2002 to December 2002. Telephone: +91 484 2452546 6. Ophthalmologist at LUKE MEMORIAL INSTITUTE, Perumbavoor September 2000 to February 2001. Telephone: +91 484 2522123 7. Resident Doctor in the Department of Paediatrics and Neonatology M.A.J. HOSPITAL, Edappally September 1997 to March 1998. Telephone: +91 484 2346996 OPHTHALMOLOGY QUALIFICATIONS AND TRAINING COURSES FRCS Ophthalmology Glasgow Passed Part III FRCS exam in February 2014. ICO Fellowship Passed the Advanced ICO examination in October 2012 (FICO) Glaucoma Fellowship Underwent two months intensive Glaucoma short-term fellowship at Aravind Eye Institute, Madurai, INDIA May 1st 2009 till June 26th 2009. -
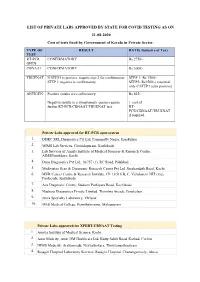
LIST of PRIVATE LABS APPROVED by STATE for COVID TESTING AS on 21-08-2020 Cost of Tests Fixed by Government of Kerala in Private Sector
LIST OF PRIVATE LABS APPROVED BY STATE FOR COVID TESTING AS ON 21-08-2020 Cost of tests fixed by Government of Kerala in Private Sector. TYPE OF RESULT RATE( Inclusive of Tax) TEST RT-PCR CONFIRMATORY Rs 2750/- OPEN CBNAAT CONFIRMATORY Rs 3000/- TRUENAT If STEP1 is positive, require step 2 for confirmation STEP 1- Rs 1500/- STEP 1 negative is confirmatory STEP2- Rs1500/-( required only if STEP1 turns positive) ANTIGEN Positive results are confirmatory. Rs 625/- Negative results in a symptomatic person require + cost of further RT-PCR/CBNAAT/TRUENAT test RT- PCR/CBNAAT/TRUENAT if required Private Labs approved for RT-PCR open system 1. DDRC SRL Diagnostics Pvt Ltd, Panampilly Nagar, Ernakulam 2. MIMS Lab Services, Govindapuram, Kozhikode 3. Lab Services of Amrita Institute of Medical Sciences & Research Centre, AIMSPonekkara, Kochi 4. Dane Diagnostics Pvt Ltd, 18/757 (1), RC Road, Palakkad 5. Medivision Scan & Diagnostic Research Centre Pvt Ltd, Sreekandath Road, Kochi 6. MVR Cancer Centre & Research Institute, CP 13/516 B, C, Vellalaserri NIT (via), Poolacode, Kozhikode 7. Aza Diagnostic Centre, Stadium Puthiyara Road, Kozhikode 8. Neuberg Diagnostics Private Limited, Thombra Arcade, Ernakulam 9. Jeeva Specialty Laboratory, Thrissur 10. MES Medical College, Perinthalmanna, Malappuram Private Labs approved for XPERT/CBNAAT Testing 1. Amrita Institute of Medical Science, Kochi 2. Aster Medcity, Aster DM Healthcare Ltd, Kutty Sahib Road, Kothad, Cochin 3. NIMS Medicity, Aralumoodu, Neyyattinkara, Thiruvananthapuram 4. Rajagiri Hospital Laboratory Services, Rajagiri Hospital, Chunangamvely, Aluva 5. Micro Health LAbs, MPS Tower, Kozhikode 6. Believers Church Medical College Laboratory, St Thomas Nagar, Kuttapuzha P.O., Thiruvalla 7. -

Prospectus 2017/18
#JOINNOW! NURSING GIVES YOU WINGS TO FLY! Prospectus 2017/18 M.Sc Nursing PB B.Sc Nursing B.Sc Nursing ASSOCIATION OF THE MANAGEMENTS OF CHRISTIAN SELF FINANCING NURSING AMCSFNCK COLLEGES OF KERALA www.amcsfnck.com Annexure - A INDEX SN Names of Member Colleges Code Total Intake Capacity B.Sc PB B.Sc M.Sc 03 Med Sur OBG Pead Psych Comty Member Colleges 1 Amala College of Nursing, Thrissur ATN 50 - 5 3 - 5 - 2 Assisi College of Nursing, Erumely, Kottayam AHN 40 - - - - - - 10 3 Bishop Benziger College of Nursing, Kollam BBN 50 - 5 3 5 3 3 Admission to 4 Canossa College of Nursing, Kannur CPN 50 - - - - - - M.Sc Nursing 5 Caritas College of Nursing, Kottayam CAN 50 25 4 4 - - 4 6 Carmel College of Nursing, Aluva, Ernakulam CLN 40 - - - - - - NURSING 11 7 College of Nursing, Nirmala Medical Centre, Muvattupuzha NMN 50 30 - - - - - Admission to 8 Holy Cross College of Nursing Adoor, Pathanamthitta HRN 40 - - - - - - GIVES YOU Post Basic 9 Holy Cross College of Nursing Kottiyam, Kollam HCN 50 35 5 4 4 2 5 WINGS TO FLY! B.Sc Nursing 10 Holy Family College of Nursing, Thodupuzha, Idukki HFN 50 20 4 - - - 4 Prospectus 2017/18 11 Jubilee Mission College of Nursing, Thrissur JMN 50 - 3 3 2 2 - 13 12 Lisie College of Nursing, Ernakulam LHN 50 30 - - - - - Admission to 13 Little Flower College of Nursing, Angamaly, Ernakulam LFN 50 20 5 5 5 5 - B.Sc Nursing 14 Little Flower College of Nursing, Payalam, Trivandrum JBN 40 - - - - - - 15 Little Lourdes College of Nursing, Kidangoor, Kottayam LDN 40 - - - - - - 14 16 Lourdes College of Nursing, Ernakulam LCN 50 50 3 3 3 5 3 General 17 Mar Sleeva College of Nursing, Palai, Kottayam MAN 60 20 6 2 - - 3 information 18 Mercy College of Nursing, Thalayolaparambu, Kottayam MUN 30 - - - - - - 19 M.O.S.C. -

Litt of N Little Flower College of Nursing
Management Trivandrum Social Service Society Management Director Little Flower Hospital Trust Fr. Cleetus Vincent Director Email: [email protected] Rev. Dr. Varghese Pottackal Principal Principal Prof. Dr. Sindhu Kuruvilla Dr. Priya Joseph Email: [email protected] Details of Contact Person Details of Contact Person Dr. Priya Joseph, Principal Prof. Dr. Sindhu Kuruvilla, Principal Little Flower College Tel: 0484-2456448 Little Flower College Mob: 9497454950 Parent Hospital Parent Hospital of Nursing Little Flower Hospital & Research Centre. of Nursing Jubilee Memorial Hospital, Palayalam, Trivandrum Little Flower College of Nursing, Little Flower Hospital Hostel Facilities Little Flower College of Nursing, Monvila, and Research Centre, Angamaly, Ernakulam - 683572 Boys: Not available / Girls: Available Kulathoor P.O., Trivandrum - 695583 Hostel Facilities Boys: Not available / Girls: Available Tel: 0484-2456448, 2675078, 2675000, Distance from nearest Railway Station Tel: 0471-2590032, 2590042, Mob: 09497454950, Fax: 0484-2452646, 2456448 2 km - Angamaly Fax: 0471-2590052 Distance from nearest Railway Station Email: [email protected], Email: [email protected] 4 km - Kazhakuttom Railway Station Distance from nearest Bus Stand Web: www.littleflowercn.com Web:www.lfhospital.org, www.lfcon.org 2 Km - Pvt. Bus Stand, Distance from nearest Bus Stand 0.5 km - KSRTC Bus stand 2 km - Sreekariyam ANGAMALY TRIVANDRUM Total Seats Govt. Seats NRI Management Open Merit Christian Total Seats Govt. Seats NRI Management Open Merit Christian Seats Community Seats Community B.Sc 50 25 - 25 15 10 B.Sc 40 20 06 14 - 14 PB B.Sc 20 10 - 10 10 - Details of the College and Parent Hospital: Little Flower College of Nursing is an institution of Trivandrum Social Service Society (TSSS) under Latin Archdiocese of Trivandrum. -

Admission Brochure of Amrita Master of Social Work, Amritapuri (2019)
MASTER OF SOCIAL WORK MASTER OF SOCIAL WORK From india’s No 1 Private University Amrita Vishwa Vidyapeetham, Amritapuri Campus United Nations UNESCO Chair in Gender Equality Educational, Scientic and and Women’s Empowerment (India) Cultural Organization MASTER OF SOCIAL WORK AMRITA VISHWA VIDYAPEETHAM Amrita Vishwa Vidyapeetham is a multi-campus, multi-disciplinary research academia that is accredited 'A' by NAAC and is ranked as one of the best research institutions in India. Amrita is spread across six campuses in three states of India - Kerala, Tamil Nadu and Karnataka, with the headquarters at Ettimadai, Coimbatore, Tamil Nadu. Amrita Vishwa Vidyapeetham continuously collaborates with top US universities including Ivy league universities and top European universities for regular student exchange programs, and has emerged as one of the fastest growing institutions of higher learning in India. The institution is managed by the Mata Amritanandamayi Math. A renowned humanitarian leader and spiritual teacher, Sri Mata Amritanandamayi is the guiding light of Amrita Vishwa Vidyapeetham. Amma’s concept of education, stress on research and commitment to instilling universal values have come together to shape Amrita Vishwa Vidyapeetham into an institution where the latest advancements and discoveries combine with compassion and service-mindedness. As Mata Amritanandamayi said in 2010 when the State University of New York honored her with an honorary Doctorate in Humane Letters: “It is Amma’s prayer that we develop the expansive-mindedness to embrace both scientific knowledge and spiritual wisdom. We can no longer afford to see these two streams of knowledge as flowing in opposite directions. In truth, they complement one another. -
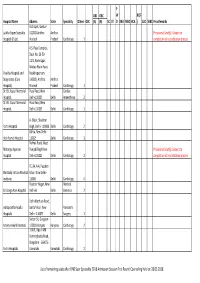
Remaining Seats After 28.03.18.Xlsx
P OBC OBC W BC(F Hospital Name Address State Speciality Others OBC (A) (B) SC ST D RBA MBC BCA ) LAC SEBC Prov.Remarks Kothapet, Guntur- Lalitha Super Specialty 522001 Andhra Andhra Provisional Seat(s): Subject to Hospital (P) Ltd. Pradesh Pradesh Cardiology 1 completion of accreditation process A.S. Raja Complex, Door No. 10-50- 11/5, Ramnagar, Waltair Main Road, Visakha Hospital and Visakhapatnam- Diagnostics (Care 530002, Andhra Andhra Hospital) Pradesh Pradesh Cardiology 1 Dr. B L Kapur Memorial Pusa Road, New Cardiac Hospital, Delhi-110005 Delhi Anaesthesia 2 Dr. B L Kapur Memorial Pusa Road, New Hospital, Delhi-110005 Delhi Cardiology 1 A- Block, Shalimar Fortis Hospital Bagh, Delhi- 110088 Delhi Cardiology 2 Okhla, New Delhi- Holy Family Hospital 110025 Delhi Cardiology 1 Rohtak Road, West Maharaja Agarsen Punjabi Bagh New Provisional Seat(s): Subject to Hospital Delhi-110026 Delhi Cardiology 2 completion of accreditation process FC-34, A-4, Paschim Shri Balaji Action Medical Vihar, New Delhi - Institute, 110063 Delhi Cardiology 4 Rajinder Nagar, New Medical Sir Ganga Ram Hospital Delhi-60 Delhi Genetics 2 Delhi Mathura Road, Indraprastha Apollo Sarita Vihar, New Paediatric Hospitals Delhi - 110076 Delhi Surgery 1 Sector 51, Gurgaon- Artemis Health Institute 122001 Haryana Haryana Cardiology 2 154/9, Opp. IIMB Bannerghatta Road, Bangalore - 560076 Fortis Hospitals Karnataka Karnataka Cardiology 2 List of remaining seats after DNB Suer Speciality 2018 Admission Session First Round Counseling held on 28.03.2018 Near Mahaveer Circle, Pumpwell, Kankanady, Indiana hospital and Manglore Karnataka- Provisional Seat(s): Subject to Heart Institute 575002 Karnataka Cardiology 1 completion of accreditation process. -
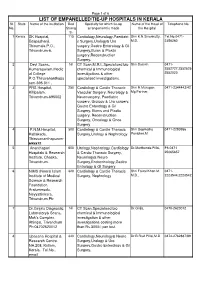
LIST OFSST Hosp.& Ph.NO
Page 1 of 6 LIST OF EMPANELLED/TIE-UP HOSPITALS IN KERALA Sl. State Name of the Institution Bed Specialty for which tie-up Name of the Head of Telephone No. No. Streng arrangement is made the Hospital th 1 Kerala SK Hospital, 115 Cardiology,Neurology,Paediatri Shri K.N.Sivankutty, Tel.No.0471- Edapazhanji, c Surgery,Urology& Uro M.D. 2356260 Thirumala P.O., surgery,Gastro Enterology & GI Trivandrum-6. Surgery,Burns & Plastic surgery,Reconstruction Surgery. 2 Devi Scans, Nil CT Scan,M.R.I.,Specialised bio Shri Suresh 0471- Kumarapuram,Medic chemical & Immunological 2552727,2552626 al College investigations & other 2552020 P.O.Thiruvananthapu specialised investigations. ram-695 011 . 3 PRS Hospital, 250 Cardiology & Cardio Thoracic Shri R.Murugan, 0471-2344443/42 Killipalam, Vascular Surgery, Neurology & Mg.Partner, Trivandrum-695002 Neurosurgery, Paediatric surgery, Urology & Uro surgery, Gastro Enterology & GI Surgery, Burns and Plastic surgery, Reconstruction Surgery, Oncology & Onco Surgery 4 P.N.M.Hospital, 300 Cardiology & Cardio Thoracic Shri Gopinatha 0471-2290956 Kattakada, Surgery,Urology & Nephrology Panicker,M. Thiruvananthapuram- 695572. 5 Ananthapuri 600 Urology,Nephrology,Cardiology Dr.Marthanda Pillai, Ph.0471- Hospitals & Research & Cardio Thoracic Surgery, 25065657 Institute, Chacka, Neurology& Neuro Trivandrum. Surgery,Endocrinology,Gastro Entrology & GI Surgery 6 NIMS (Nooral Islam 320 Cardiology & Cardio Thoracic Shri Faizal Khan M, 0471- Institute of Medical Surgery, Nephrology M.D., 2223544,2223542 Science & Research Foundation, Aralummodu, Neyyattinkara, Trivandrum,Ph: 7 Dr.Girija's Diagnostic Nil CT Scan,Specialised bio Dr.Girija, 0470-2620012 Laboratory& Scans, chemical & Immunological Mak's Complex, investigation & other Attingal, Trivandrum investigations costing more Ph:04702620012 than Rs.3000/- per test. -

Blde University Shri Bm Patil Medical College Hosp
Blde University Shri Bm Patil Medical College Blde University Shri Bm Patil Medical College Hosp Karnataka Vijayapura Hosp Bangramma Sajjan Campus Ashram Road Bijapur 586103 Tanga Hospital A 89 Bahavasarnagar Jail Darga Road Bijapur 586103 Karnataka Vijayapura ANNAPURNA MULTI SPECIALTY HOSPITAL OPPOSITE KARIGOUDAR LABORATORY GODBOLE Karnataka Vijayapura MALA TAJ BAVADI ROAD VIJAYAPURA Vaibhav orthopaedic And Dental Care Hospital Mukund Nagar Station Road Near Madhuvan Hotel Karnataka Vijayapura Bijapur Kidney Foundation Bijapur Kidney Foundation Near Mugalkhod Math Karnataka Vijayapura Solapur Road Bijapur 586101 alnabi hospital Near Zandakatta J M Road Vijaypur Karnataka Vijayapura Mudhol Hospital Allapur Base JM Road Bijapur Karnataka Vijayapura Choudhari Hospital Choudhari Hospital Near Lic Office Shikarkhana Road Karnataka Vijayapura Bijapur 586104 Al Ameen Medical College Hospital Al Ameen Medical College Hospital Al Ameen Campus Karnataka Vijayapura Athani Road Vijayapur 586108 Shri Bhagyavanti Multispeciality Hospital And Rc Shri Bhagyavanti Multispeciality Hospital And Rc 8 16 Karnataka Vijayapura Mallikarjun Nagar Near Ganesh Nagar Bus Stop Bijapur 586109 SHRI SAI HOSPITAL 32 33 VIJAYPUR BENGALURU HIGHWAY JUNCTION Karnataka Vijayapura DR G R BHAT HOSPITAL TALIKOT Dr G R BHAT M G Road Opp Town Municipal Karnataka Vijayapura Corporation Talikote Huda Trust Hospital Kerala Alappuzha Sreekantapuram Hospital Kerala Alappuzha St.Sebastian Hospital Arthunkal Kerala Alappuzha Sahrudaya Hospital Kerala Alappuzha Maha Jubilee Memorial Hospital -

Press Release Little Flower Hospital Trust
Press Release Little Flower Hospital Trust February 10, 2021 Rating Amount Facilities/Instruments Ratings Rating Action (Rs. crore) CARE BB+; Stable; Revised from CARE BBB-; Stable ISSUER NOT COOPERATING* (Triple B Minus; Outlook: Stable) and Long Term Bank Facilities 25.38 (Double B Plus; Outlook: Stable moved to ISSUER NOT COOPERATING ISSUER NOT COOPERATING*) category 25.38 (Rs. Twenty-Five Total Bank Facilities Crore and Thirty-Eight Lakhs Only) Details of instruments/facilities in Annexure-1 Detailed Rationale & Key Rating Drivers CARE has been seeking information from Little Flower Hospital Trust to monitor the rating vide e-mail communications/letters dated December 03, 2020, December 08, 2020, December 17, 2020 , January 04, 2021, January 14, 2021 among others and numerous phone calls. However, despite our repeated requests, the trust has not provided the requisite information for monitoring the ratings. In line with the extant SEBI guidelines, CARE has reviewed the rating on the basis of the best available information which however, in CARE’s opinion is not sufficient to arrive at a fair rating. The rating on Little Flower Hospital Trust’s bank facilities will now be denoted as CARE BB+; Stable ; ISSUER NOT COOPERATING*. Users of this rating (including investors, lenders and the public at large) are hence requested to exercise caution while using the above rating. The rating have been revised on account of absence of information on entity’s financial and operational performance and other critical data related to the business of the entity post the last review. Detailed description of the key rating drivers At the time of last rating on April 6, 2020 the following were the rating strengths and weaknesses: Key Rating Strengths Improvement in the overall financial profile marked by growth in gross receipts, improved SBID margins, capital structure and debt coverage indicators The gross receipts of the trust increased by ~16% from Rs.109.40 crore in FY18 to Rs.127.04 crore in FY19.