BLEACH (Sodium Hypochlorite Solution)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
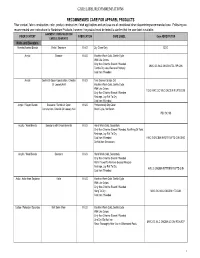
Care Label Recommendations
CARE LABEL RECOMMENDATIONS RECOMMENDED CARE FOR APPAREL PRODUCTS Fiber content, fabric construction, color, product construction, finish applications and end use are all considered when determining recommended care. Following are recommended care instructions for Nordstrom Products, however; the product must be tested to confirm that the care label is suitable. GARMENT/ CONSTRUCTION/ FIBER CONTENT FABRICATION CARE LABEL Care ABREVIATION EMBELLISHMENTS Knits and Sweaters Acetate/Acetate Blends Knits / Sweaters K & S Dry Clean Only DCO Acrylic Sweater K & S Machine Wash Cold, Gentle Cycle With Like Colors Only Non-Chlorine Bleach If Needed MWC GC WLC ONCBIN TDL RP CIIN Tumble Dry Low, Remove Promptly Cool Iron If Needed Acrylic Gentle Or Open Construction, Chenille K & S Turn Garment Inside Out Or Loosely Knit Machine Wash Cold, Gentle Cycle With Like Colors TGIO MWC GC WLC ONCBIN R LFTD CIIN Only Non-Chlorine Bleach If Needed Reshape, Lay Flat To Dry Cool Iron If Needed Acrylic / Rayon Blends Sweaters / Gentle Or Open K & S Professionally Dry Clean Construction, Chenille Or Loosely Knit Short Cycle, No Steam PDC SC NS Acrylic / Wool Blends Sweaters with Embelishments K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed, No Wring Or Twist Reshape, Lay Flat To Dry Cool Iron If Needed HWC S ONCBIN NWOT R LFTD CIIN DNID Do Not Iron Decoration Acrylic / Wool Blends Sweaters K & S Hand Wash Cold, Separately Only Non-Chlorine Bleach If Needed Roll In Towel To Remove Excess Moisture Reshape, Lay Flat To Dry HWC S ONCBIN RITTREM -

Care Labelling Guide
Care Labelling Guide This chart is for guidance only and should be read in conjunction with the ACCC Care labelling for Clothing Guide which specifies the mandatory standards for care labelling based on the Australian/New Zealand Standard (AS/NZS) 1957:1998, Textiles—Care labelling with variations and additions made by Consumer Protection Notice No. 25 of 2010. In Australia, care instructions must be permanently attached to articles, be written in English, and be appropriate for the care of the article. Additional symbols may be used to clarify the instructions but symbols alone are not sufficient. As a minimum, laundering instructions include (in order) four symbols: washing, bleaching, drying, and ironing. Drycleaning instructions include one symbol. Professional Wet Cleaning symbols are included for information only as they are not currently recognised in Australia. Washing The washtub symbol tells you how the garment should be washed. The number inside the washtub is the maximum wash temperature. A bar underneath the washtub indicates that the garment should have mild treatment or washed using the Permanent Press setting. Two lines underneath the washtub indicate gentle or delicate treatment. Normal Permanent Press Delicate / Gentle Hand Wash 95°c 70°c 60°c 50°c 40°c 30°c Do Not Wring Do Not Wash Bleaching The triangle symbol gives information about whether it is safe to bleach the garment. Any Bleach (when Only non-chlorine Do Not Bleach needed) bleach (when needed) Drying Tumble Dry - Cycles Normal Permanent Press Delicate / Gentle Do not tumble dry © 2013, Drycleaning Institute of Australia Limited Tumble Dry - Settings Any Heat High Medium Low No Heat / Air Line Dry - Hang to dry Drip Dry Dry Flat In the shade Do not dry Ironing The Iron symbol provides information on how the garment should be ironed. -

Women in Industry
GORNEM. LAW I.IBIIARY -Gxj; Kf Z5SS aiorttfU ICam ^t\\\xsx\ Eibtar^ Cornell University Library KF3555.A5B81 Women fn industry; decision of the United 3 1924 017 572 391 Cornell University Library The original of tiiis book is in tine Cornell University Library. There are no known copyright restrictions in the United States on the use of the text. http://www.archive.org/details/cu31924017572391 WOMEN IN INDUSTRY DECISION OF THE UNITED STATES SUPREME COURT IN CURT MULLER VS. STATE OF OREGON UPHOLDING THE CONSTITUTIONALITY OF THE OREGON TEN HOUR LAW FOR WOMEN AND BRIEF FOR THE STATE OF OREGON BY LOUIS D. BRANDEIS Assisted by JOSEPHINE GOLDMARK Fublication Secretary National Consumers' League REPRINTED FOR THE NATIONAL CONSUMERS' LEAGUE 105 East 22d Strest, New York City ^"io^^ol TABLE OF CONTENTS Pages Laws of the Several States in Force Limiting the Hours op Labor op Adult Women 1-8 ARGUMENT 9, 10 FIRST PART — Legislation Restricting the Hours of Labor for Women 11-17 I. THE FOREIGN LEGISLATION 11-15 n. THE AMERICAN LEGISLATION 16, 17 PART SECOND — The World's Experience upon which the Legislation Lvmitmg the Hours of Labor for Women is Based 18-112 I. THE DANGERS OF LONG HOURS 18-55 A. Causes 18-27 (1) Physical Differences between Men and Women . 18-23 (2) The New Strain in Manufacture 24-27 B. Bad Eppect op Long Hours on Health 28-46 28-36 (1) General Injuries from Long Hours ., (2) Specific Evil Effects on Childbirth and Female Functions 36-42 C. Bad Eppect op Long Hours on Safety 42-44 D. -

CHEMICALS Laundry Detergent/Bleach
Laundry Detergent/Bleach A A. INSTITUTIONAL HEAVY-DUTY DETERGENT CCP A concentrated blend of cleaning agents which will emulsify grease and dirt in either hard or soft water with excellent results. This synthetic detergent formula contains water softening, fabric brighteners, grease emulsifiers and soil suspending agents. 27600119 P550262 50 lb. 1/bx. B. ALL® POWDER LAUNDRY DETERGENT JOHNSON DIVERSEY ALL uses Stainlifters™ to safely lift dirt and stains. All Ultra® Powder Laundry Detergent 15200115 2979267 (100) 2 oz. Box 100/cs. B 15200176 2979304 150 Use 1/ea. All® Concentrated Powder Laundry Detergent 15200112 2979216 50 lb. Concentrated 1/ea. C C. SURF ULTRA® POWDER JOHNSON DIVERSEY LAUNDRY DETERGENT Surf® with Active Oxygen formula lifts dirt and odors while leaving laundry smelling fresh. 15200158 2979814 2 oz. 100/cs. D. TIDE® POWDER LAUNDRY DETERGENT PROCTER & GAMBLE Tide with bleach cleans so well…it’s the only detergent that kills 99.9% of bacteria, including Staphylococcus Aureus, Klebsiella Pneumoniae, E. coli and Salmonella Choleraesuis. Use D one full scoop as directed. Contains a unique activated bleaching system. Removes many tough stains and whitens whites. Helps keep colors bright. Non-phosphate. Harmless to septic tanks. EPA Registration No. 3573-56. 16902370 32370 33 oz. 15/cs. CHEMICALS w/Bleach Contains a unique active bleaching system. Removes many tough stains and whitens whites. Helps keep colors bright. No phosphate. Harmless to septic tanks. Kills 99.9% of bacteria (effective against Staphylococcus Aurius and Klebsiella Pneumoniae; use as directed). 16901801 02370 4 oz. 100/cs. 16900095 40026 8 oz. 14/cs. 16907330 84907330 33 oz. -

Changes in Mechanical Properties of Denim Due to Different Washing Processes
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056 Volume: 04 Issue: 10 | Oct -2017 www.irjet.net p-ISSN: 2395-0072 Changes in Mechanical Properties of Denim Due to Different Washing Processes Israt Zebin Ansari Dept. of Textile Engineering, Southeast University, Dhaka, Bangladesh ---------------------------------------------------------------------***--------------------------------------------------------------------- Abstract – The paper is on “changes in mechanical 2. MATERIAL & METHODOLOGY properties of denim due to different washing processes” here 100% cotton denim fabrics were selected to carry out the 2.1 Materials: experiment, and different washing process; Bleach Wash, Enzyme wash, PP wash applied on 100% cotton twill, weave 2.1.1 Fabric 2/1, construction 80 × 64 / 10 × 9, indigo dyed denim fabric leg panels as per lab standard recipe. Pretreatment was All specimens are made from one shell fabric 100% cotton Desizing and after treatment is silicon softener. After washing twill left hand twill, weave 2/1, construction 80 × 64 / 10 × different samples from different washing are going to express 9, indigo dyed denim fabric , GSM-225 is used in this work. different mechanical properties. Finding out the different Leg panels were made using the stated denim fabric. We expression of denim for different wash is the major part of that collected it from CIPL-Epic group. research. 2.1.2 Chemicals Key Words: Denim fabrics, Twill, Enzyme, Bleach, PP, Desizing, softener, Mechanical properties. Detergent (Hostapur, BASF, Germany), Acetic acid (china), desizing agent (Luzyme, BASF, Germany), softener (silicon 1. INTRODUCTION softener, china), Cryltane DTS 40 (china), Sodium metabisulfite (neutralization agent).Enzyme (acid enzyme, Denim is one of the world’s oldest fabric’s which is most Bangladesh), Bleaching agent ((Bleach KCI, India), KMNO4 commonly associated with jeans. -

Tackling Your Laundry: Let’S Talk Products! Welcome to the Ever Changing World of Laundry Detergents, Stain Removal Products, Fabric Softeners and Dryer Sheets
FRM-AP.017 Tackling Your Laundry: Let’s talk products! Welcome to the ever changing world of laundry detergents, stain removal products, fabric softeners and dryer sheets. Some of today’s choices can become overwhelming. Whatever the product choice, the information in this There are publication should help you keep your laundry room safe. three types of energy Choosing the right laundry detergent Laundry detergents come in many forms, each having its own benefits. needed for Select the form that meets your specific need. good cleaning General purpose detergents Full-strength detergents can be used with many types of fabrics/textiles. results: These products include: Chemical — • High Efficiency (HE) detergents provided by • Ultra detergents detergents • Single-use detergents (pods) and laundry • Fragrance or dye-free detergents • Bar soap aids; • Combination detergents Thermal — provided by Light duty detergents These detergents can be used when washing fabrics by hand or in your the water washing machine. They are used primarily for delicate fabrics, such as those temperature; requiring special care, or lightly-soiled items; ideal for hand washing baby Mechanical clothes. These products include: — provided • Liquid Detergents by the • Powdered Detergents machine agitation. All three need to be considered for best results! Liquid, powders and enzyme detergents Liquid detergents work at all temperatures and are easy to use for pretreating stains. Liquid detergents have an ingredient that helps soften hard water minerals. Ground in dirt and clay are best lifted from soiled laundry with powder detergents using warm to hot water. Protein and oil-based stains are best removed using enzyme detergents. -

FANTASTIK® ALL PURPOSE CLEANER with BLEACH (DIN 02248237) Version 1.1 Print Date 01/09/2018
Safety Data Sheet classification according to Canadian Hazardous Products Regulation FANTASTIK® ALL PURPOSE CLEANER WITH BLEACH (DIN 02248237) Version 1.1 Print Date 01/09/2018 Revision Date 01/09/2018 SDS Number 350000004371 1. PRODUCT AND COMPANY IDENTIFICATION Product information Product name : FANTASTIK® ALL PURPOSE CLEANER WITH BLEACH (DIN 02248237) Recommended use : Speciality CleanerSpeciality Cleaner Restrictions on use : Use only as directed on label Manufacturer, importer, : S.C. Johnson and Son, Limited supplier 1 Webster Street Brantford ON N3T 5R1 Telephone : +1-800-558-5566 Emergency telephone : 24 Hour Transport & Medical Emergency Phone (866) 231-5406 number 24 Hour International Emergency Phone (952) 852-4647 24 Hour Canadian Transport Emergency Phone (CANUTEC) (613) 996-6666 2. HAZARDS IDENTIFICATION Classification of the substance or mixture Globally Harmonized System (GHS) Classification Hazard classification Hazard category Hazards identification Skin irritation Category 2 Causes skin irritation. Eye irritation Category 2A Causes serious eye irritation. Labelling Hazard symbols Exclamation mark Signal word Warning Hazard statements 1/16 Safety Data Sheet classification according to Canadian Hazardous Products Regulation FANTASTIK® ALL PURPOSE CLEANER WITH BLEACH (DIN 02248237) Version 1.1 Print Date 01/09/2018 Revision Date 01/09/2018 SDS Number 350000004371 Causes skin irritation. Causes serious eye irritation. Precautionary statements If medical advice is needed, have product container or label at hand. Keep out of reach of children. Read label before use. Specific treatment (see supplemental first aid instructions on this label). IF ON SKIN: Wash with plenty of soap and water. If skin irritation occurs: Get medical advice/ attention. IF IN EYES: Rinse cautiously with water for several minutes. -

Knolltextiles Cleaning Information
Cleaning Information Regular Maintenance It is important to vacuum upholstery regularly to remove the surface dust that builds up and slowly contributes to a greying effect that diminishes the clarity of the original color. Please note that vacuuming should be done with the proper attachments to avoid breaking down the fibers and contributing to “pilling.” In addition to regular vacuuming, a professional cleaner should come in at least once or twice a year to thoroughly clean the fabrics and remove the soiling that a normal vacuum cleaner cannot reach. The expense of this service is small compared to the reward of seating that looks good as new after a lot of use. Cleaning Codes W: Water-based cleaning agents or foam may be used for cleaning this fabric. S: Only mild, pure water-free dry cleaning solvents may be used for cleaning this fabric. W-S: Water-based cleaning agents and foam or mild, waterfree solvents may be used for cleaning this fabric. W Bleach: Water-based or foam cleaning agents or diluted household bleach may be used for cleaning this fabric. W-S Bleach: Clean with water or solvent-based cleaning agents or diluted household bleach. X: This fabric should be vacuumed or brushed lightly to remove soil. Warning: Do not use water-based foam or liquid cleaning agents of any type on this fabric. Cleaning vs Sanitizing vs Disinfecting Cleaning, disinfecting and sanitizing are often used synonymously, but they are not the same thing. Cleaning removes the visible foreign matter from a surface. Disinfecting, when done according to the instructions of a suitable cleaner, kills all bacteria and viruses present, while sanitizing reduces the level of bacteria and viruses present. -

Sunbrella Stain Chart
AWNINGS care and cleaning One of the best ways to keep Sunbrella® fabrics Heavy cleaning for Applying 303 High Tech looking good and to delay the need for deep or vigorous cleaning is to hose fabrics off on a monthly stubborn stains and mildew Fabric Guard basis with clear water. This practice will help prevent dirt from becoming deeply embedded in the fabric Sunbrella fabric does not promote mildew growth, 303 should be applied to Sunbrella fabrics after each and eliminate the need for more frequent vigorous however, mildew may grow on dirt and other foreign thorough cleaning, which typically removes the original finish cleaning. In most environments, a thorough cleaning substances that are not removed from the fabric. To and reduces the fabric’s water repellency. will be needed every two to three years. clean mildew, or other stubborn stains: • Clean Sunbrella fabric, using one When it’s time for a thorough cleaning, Sunbrella • Eight ounces (1 cup) of chlorine bleach. of the cleaning methods. fabrics can be cleaned while still on an awning frame • Two ounces (1/4 cup) of mild • Allow Sunbrella to completely air dry. or, size permitting, they can be removed for cleaning soap and/or detergent. • Apply 303 Fabric Guard in a well ventilated in a washing machine. • One gallon of water. area following instructions on the container. • Clean with soft bristle brush. • Apply 303 in a thin, even coat and When cleaning Sunbrella fabrics, it is important to • Allow mixture to soak into fabric allow fabric to dry completely. observe the following: for up to 15 minutes. -

“I” Cards Copy This Page to Play the Game
“I” Cards Copy this page to play the game. Black Pants Bathroom Rug White Towels 100% polyester; Red Bathing Suit 85% nylon 100% nylon 100% cotton double knit 15% spandex greasy spots No care label Care label Care label Care label Machine wash with Machine wash warm Machine wash cold, like colors, before darks separately, hang dry. Do not first use, tumble dry. tumble dry, remove bleach, wash Do not bleach promptly and do dark colors separately. colored towels. not bleach. White Dress Shirt Red Beach Towel Green Sleeping Bag Plaid Slacks 80% polyester/ 65% Cotton 100% Cotton 100% Cotton 30% cotton 35% polyester “guaranteed to bleed” Care label Care label Machine wash Care label Machine wash with seperately on delicate Care label like colors (deep Machine wash cold, Machine wash warm, cycle in warm eater. gentle cycle, colors bleed), before Dry at a low wash separately. first use, tumble dry. temperature. Remove Tumble dry low, tumble dry low, do not bleach and Do not bleach from dryer immediately warm iron. at end of drying cycle. use warm iron. colored towels. Do not dry clean. Blue and White Purple Warm-up Suit Blue Baby Clothes Football Jersey Brown Jacket 100% acrylic 100% Cotton 100% wool 100% nylon, Food stained grass stained. Care label Care label Care label Machine wash cold, Care label Machine wash with Dry clean. wash with like colors. Machine wash warm like colors. Only non- No bleach. Tumble gentle cycle, tumble chlorine bleach when dry on low. dry on low and use a needed. Tumble dry Cool iron. -

ASI CAMPUS Laundry Solutions RA RALLY
How to Wash LAUNDRY 101 How to Dry Service Hang out and learn about laundry How to wash, how to dry, and service reporting made easy Your Laundry Program • Social distancing in the laundry room • How to wash and dry • Let’s talk about soap • Placing a service request • Common laundry mistakes • Fun student activities and education • Connect with us on Facebook, Twitter, and Instagram Your Safety is Our Top Priority Do not enter Limit visits to if you are loading & unloading experiencing of washers and symptoms or dryers only. feeling sick. Limit the number Wear a mask each of people in the time you visit the laundry room laundry room. at one time. Fold clothes in your Keep a six foot dorm room distance between and not in the you and others. laundry room. Washing Instructions How to get the best wash results It all starts with sorting Sort Clothes by Color • Sort laundry according to color, grouping dark, medium, and light colors separately. • Wash deep colored clothing by themselves for the first few washes. Sort Clothes by Fabric Type • Wash heavier items, like towels, separately from lighter weight clothes to prevent damage to finer fabrics. • Separate items that shed lint from microfiber, corduroy or other fabrics that attract lint. Washing Instructions How to get the best wash results Easy Wash Simple Dry 1. Add detergent or 1. Clean lint screen for simply toss in a laundry maximum air flow pod 2. Select temperature 2. Load washer, leaving 3. Select cycle about five inches of space from the top 4. -

Dry Bleach 6%
DryDry BleachBleach 6%6% Dry Chlorinated Bleach for Whites Dry Bleach is a highly chlorinated product with fast bleaching action. It is used for normal bleaching of white linen in commercial and institutional laundry machines. Powdered bleaches are safer than liquids. They release the active bleaching agent into the wash more slowly to create a more balanced bleaching effect. Traditional liquid bleaches may shock the fabric and sometimes create a yellowing effect. Dry Bleach 6% has a unique combination of ingredients to safely bleach all washable white laundry fabrics whiter and brighter and remove stains, whether you are washing in hot, warm, or cold water. Dry Bleach 6% is particularly effective in removing stains from cotton and linen fabrics. Dry Bleach 6% is free-flowing and offers increased versatility and ease of handling. It can be added in dry form to automatic washers or to the supply compartment of automatic feeding equipment. Dry Bleach 6% dissolves readily and completely, leaves no residues, and does not cake on equipment surfaces. Since Dry Bleach 6% is a dry powder, it remains stable during storage with no loss of chlorine or bleaching power. • Highly Concentrated to Reduce • Dissolves Quickly when Added to the Inventory and Usage Cost Wash • Superior Whitening Properties for White • Provides Rapid Stain Removal Laundry Usage Directions: Keep dry. Replace cover after use. Add appropriate amount to wash water with detergent. Safety & Hazards Dry Bleach 6% 0 Dilution Guidelines 2 1 Usage Dilution Normal Usage 3 - 9 Ounces per 100 lb Load Wear Protective Eye & Face Protection and Chemical- Resistant Gloves While Using Dry Bleach 6% Consult SDS for Further Safety Precautions DOT Shipping Name: Not Regulated Technical Information: Appearance: Free Flowing White Powder Odor: Chlorine Bleach pH: >9 Foam: Non-Foaming Associated Products: Warning: Some Fabrics May Need Special Attention.