Unresolved Issues in Theories of Autoimmune Disease Using Myocarditis As a Framework
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Picture of the Month
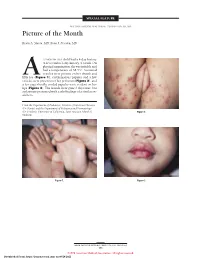
SPECIAL FEATURE SECTION EDITOR: WALTER W. TUNNESSEN, JR, MD Picture of the Month Kevin A. Slavin, MD; Ilona J. Frieden, MD 15-MONTH-OLD child had a 4-day history of fever and a 1-day history of a rash. On physical examination she was irritable and had a temperature of 38.3°C. Scattered vesicles were present on her thumb and Afifth toe (Figure 1), erythematous papules and a few vesicles were present over her perineum (Figure 2), and a few superficially eroded papules were evident on her lips (Figure 3). The lesions were gone 3 days later, but a playmate presented with early findings of a similar ex- anthem. From the Department of Pediatrics, Division of Infectious Diseases (Dr Slavin) and the Department of Pediatrics and Dermatology (Dr Frieden), University of California, San Francisco School of Figure 2. Medicine. Figure 1. Figure 3. ARCH PEDIATR ADOLESC MED/ VOL 152, MAY 1998 505 ©1998 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/28/2021 Denouement and Discussion Hand-Foot-and-Mouth Disease Figure 1. Vesicles are present on the thumb and fifth toe. foot-and-mouth disease).1,2,5-9 The lesions on the but- tocks are of the same size and typical of the early forms Figure 2. Multiple erythematous papules and a few scattered vesicles are of the exanthem, but they are not frequently vesicular present over the perineum. in nature. Lesions involving the perineum seem to be more Figure 3. Superficially eroded papules are present on the lips. common in children who wear diapers, suggesting that friction or minor trauma may play a role in the develop- ment of lesions. -

Hand, Foot, and Mouth Disease (Coxsackievirus) Fact Sheet
Hand, Foot, and Mouth Disease (Coxsackievirus) Fact Sheet Hand, foot, and mouth disease is caused by one of several types of viruses Hand, foot, and mouth disease is usually characterized by tiny blisters on the inside of the mouth and the palms of the hands, fingers, soles of the feet. It is commonly caused by coxsackievirus A16 (an enterovirus), and less often by other types of viruses. Anyone can get hand, foot, and mouth disease Young children are primarily affected, but it may be seen in adults. Most cases occur in the summer and early fall. Outbreaks may occur among groups of children especially in child care centers or nursery schools. Symptoms usually appear 3 to 5 days after exposure. Hand, foot, and mouth disease is usually spread through person-to-person contact People can spread the disease when they are shedding the virus in their feces. It is also spread by the respiratory tract from mouth or respiratory secretions (such as from saliva on hands or toys). The virus has also been found in the fluid from the skin blisters. The infection is spread most easily during the acute phase/stage of illness when people are feeling ill, but the virus can be spread for several weeks after the onset of infection. The symptoms are much like a common cold with a rash The rash appears as blisters or ulcers in the mouth, on the inner cheeks, gums, sides of the tongue, and as bumps or blisters on the hands, feet, and sometimes other parts of the skin. The skin rash may last for 7 to 10 days. -

Alternatively Activated Macrophages in Infection and Autoimmunity
NIH Public Access Author Manuscript J Autoimmun. Author manuscript; available in PMC 2010 November 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: J Autoimmun. 2009 ; 33(3-4): 222±230. doi:10.1016/j.jaut.2009.09.012. Alternatively activated macrophages in infection and autoimmunity DeLisa Fairweathera,* and Daniela Cihakovab a Department of Environmental Health Sciences, Johns Hopkins University, Bloomberg School of Public Health, Baltimore, Maryland 21205, USA b Department of Pathology, Johns Hopkins University, School of Medicine, Baltimore, Maryland 21205, USA Abstract Macrophages are innate immune cells that play an important role in activation of the immune response and wound healing. Pathogens that require T helper-type 2 (Th2) responses for effective clearance, such as parasitic worms, are strong inducers of alternatively activated or M2 macrophages. However, infections such as bacteria and viruses that require Th1-type responses may induce M2 as a strategy to evade the immune system. M2 are particularly efficient at scavenging self tissues following injury through receptors like the mannose receptor and scavenger receptor-A. Thus, M2 may increase autoimmune disease by presenting self tissue to T cells. M2 may also exacerbate immune complex (IC)-mediated pathology and fibrosis, a hallmark of autoimmune disease in women, due to the release of profibrotic factors such as interleukin (IL)-1β, transforming growth factor-β, fibronectin and matrix metalloproteinases. We have found that M2 comprise anywhere from 30% to 70% of the infiltrate during acute viral or experimental autoimmune myocarditis, and shifts in M2 populations correlate with increased IC-deposition, fibrosis and chronic autoimmune pathology. -

Treatment of Patients with Malignant Lymphomas with Monoclonal Antibodies
Bone Marrow Transplantation (2000) 25, Suppl. 2, S50–S53 2000 Macmillan Publishers Ltd All rights reserved 0268–3369/00 $15.00 www.nature.com/bmt Treatment of patients with malignant lymphomas with monoclonal antibodies H Tesch, A Engert, O Manzke, V Diehl and H Bohlen Klinik I fuer Innere Medizin, Universitaet zu Koeln, Koeln, Germany Summary: Results and discussion Malignant lymphomas represent a heterogenous group of B and T cell-derived malignancies. Most lymphomas Native monoclonal antibodies are sensitive to chemo- and radiotherapy, however many patients will eventually relapse. Immunothera- Since the first description of therapy using monoclonal anti- peutic approaches including monoclonal antibodies, bodies in 1979, several phase I and II trials have been cytokines or vaccination approaches may offer an alter- initiated to evaluate both safety and antitumoral activity of native treatment of chemotherapy-resistant residual this approach. Native MoAbs can kill a tumor cell through cells especially in cases with low tumor burden or various mechanisms including complement activation, anti- residual disease following chemo- or radiotherapy. body-dependent cellular cytotoxicity (ADCC), phago- Monoclonal antibodies have been successfully applied in cytosis of antibody-coated tumor cells, inhibition of cell their native form, or coupled with radioisotopes or tox- cycle progression, and induction of apoptosis.2,3 Alterna- ins to selectively destroy lymphoma cells and promising tively, MoAbs can eliminate a tumor cell by inhibiting results in early clinical trials have been obtained. Alter- growth factor receptors or molecules involved in signal natively, bispecific antibodies and idiotypic vaccination transduction and cell proliferation. strategies are used to target autologous T cells to elimin- The group of R Levy at Stanford reported on promising ate lymphoma cells. -

Anti-Idiotype Antibody Generation and Application in Antibody Drug Discovery
Anti-idiotype Antibody Generation and Application in Antibody Drug Discovery Liusong Yin, PhD Senior Scientist, Group Leader Antibody Discovery, Antibody Department, GenScript [email protected] Apr 21st, 2016 Presentation Overview Anti-idiotype antibody introduction 1 2 Anti-idiotype antibody application 3 Anti-idiotype antibody development 4 Anti-idiotype antibody case study Make Research Easy 2 Structural overview of antibodies PDB ID: 1HZH Liusong Yin, 2014, A Dissertation Make Research Easy 3 Antibody ‘-types’ Isotype (species specific)– the phenotypic variations in the constant regions of the heavy and light chains Allotype (animal specific)– the genetically determined difference in antibodies between individuals in the same species, mainly a couple AA differences in constant region Idiotype (antigen specific)– the antigen binding specificity defined by the distinctive sequence in the variable region of antibodies Make Research Easy 4 ‘-topes’ in anti-idiotype antibodies (anti-IDs) Idiotope – the antigenic determinants in or close to the complementarity determining region (CDR) in variable region Epitope Paratope Paratope – the part of an Ab that recognizes an antigen, the antigen-binding site of an Ab Epitope – the part of the antigen to which the paratope binds Anti-IDs – anti-idiotype antibodies which recognize the shared feature of idiotopes Make Research Easy 5 Different types of Anti-IDs Antigen-blocking Non-blocking Complex-specific Anti-ID Drug Target Anti-ID Anti-ID Antibody drug Antibody drug Antibody drug -

In the United States Court of Federal Claims OFFICE of SPECIAL MASTERS Filed: July 28, 2020
In the United States Court of Federal Claims OFFICE OF SPECIAL MASTERS Filed: July 28, 2020 * * * * * * * * * * * * * * * * MICHAEL PAVAN, next friend of * J.P., a minor, * PUBLISHED * Petitioner, * No. 14-60V * v. * Special Master Gowen * SECRETARY OF HEALTH * Entitlement; Significant AND HUMAN SERVICES, * Aggravation; Varicella; * Chronic Inflammatory Respondent. * Demyelinating Polyneuropathy * * * * * * * * * * * * * * * * (“CIDP”). Scott W. Rooney, Nemes Rooney P.C., Farmington Hills, MI, for petitioner. Kyle E. Pozza, United States Department of Justice, Washington, DC, for respondent. DECISION1 On January 24, 2014, Michael Pavan (“petitioner”), as next friend of J.P., a minor, filed a petition in the National Vaccine Injury Compensation Program.2 Petitioner alleges that as a result of J.P. receiving the varicella vaccination on January 28, 2011, he suffered a significant aggravation of his Chronic Inflammatory Demyelinating Polyneuropathy (“CIDP”). Amended Petition at ¶¶ 4, 5, & 16 (ECF No. 26); Petitioner’s (“Pet.”) Post-hearing Brief at 2 (ECF No. 151). Based on a full review of the evidence and testimony presented, I find that petitioner has not established by a preponderance of the evidence that the varicella vaccination significantly aggravated J.P.’s CIDP and therefore, compensation must be denied and the petition dismissed. 1 In accordance with the E-Government Act of 2002, 44 U.S.C. § 3501 (2012), because this opinion contains a reasoned explanation for the action in this case, this opinion will be posted on the website of the United States Court of Federal Claims. This means the opinion will be available to anyone with access to the internet. As provided by 42 U.S.C. -

In Kawasaki Syndrome Anti-Human Cardiac Myosin Autoantibodies
Anti-Human Cardiac Myosin Autoantibodies in Kawasaki Syndrome Madeleine W. Cunningham, H. Cody Meissner, Janet S. Heuser, Biagio A. Pietra, David K. Kurahara and Donald Y. This information is current as M. Leung2 of September 29, 2021. J Immunol 1999; 163:1060-1065; ; http://www.jimmunol.org/content/163/2/1060 Downloaded from References This article cites 34 articles, 19 of which you can access for free at: http://www.jimmunol.org/content/163/2/1060.full#ref-list-1 Why The JI? Submit online. http://www.jimmunol.org/ • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average by guest on September 29, 2021 Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 1999 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Anti-Human Cardiac Myosin Autoantibodies in Kawasaki Syndrome1 Madeleine W. Cunningham,* H. Cody Meissner,† Janet S. Heuser, * Biagio A. Pietra,§ David K. Kurahara,‡ and Donald Y. M. Leung2¶ Kawasaki syndrome (KS) is the major cause of acquired heart disease in children. Although acute myocarditis is observed in most patients with KS, its pathogenesis is unknown. -

A Guide to Clinical Management and Public Health Response for Hand, Foot and Mouth Disease (HFMD)
A Guide to Clinical Management and Public Health Response for Hand, Foot and Mouth Disease (HFMD) WHO Western Pacific Region PUBLICATION ISBN-13 978 92 9061 525 5 A Guide to Clinical Management and Public Health Response for Hand, Foot and Mouth Disease (HFMD) WHO Library Cataloguing in Publication Data A Guide to clinical management and public health response for hand, foot and mouth disease (HFMD) 1. Hand, foot and mouth disease – epidemiology. 2. Hand, foot and mouth disease – prevention and control. [ ii ] 3. Disease outbreaks. 4. Enterovirus A, Human. I. Regional Emerging Disease Intervention Center. ISBN 978 92 9061 525 5 (NLM Classification: WC 500) © World Health Organization 2011 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). For WHO Western Pacific Regional Publications, request for permission to reproduce should be addressed to the Publications Office, World Health Organization, Regional Office for the Western Pacific, P.O. Box 2932, 1000, Manila, Philippines, (fax: +632 521 1036, e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Myocarditis in Humans and in Experimental Animal Models
REVIEW published: 16 May 2019 doi: 10.3389/fcvm.2019.00064 Myocarditis in Humans and in Experimental Animal Models Przemysław Błyszczuk 1,2* 1 Department of Clinical Immunology, Jagiellonian University Medical College, Cracow, Poland, 2 Department of Rheumatology, Center of Experimental Rheumatology, University Hospital Zurich, Zurich, Switzerland Myocarditis is defined as an inflammation of the cardiac muscle. In humans, various infectious and non-infectious triggers induce myocarditis with a broad spectrum of histological presentations and clinical symptoms of the disease. Myocarditis often resolves spontaneously, but some patients develop heart failure and require organ transplantation. The need to understand cellular and molecular mechanisms of inflammatory heart diseases led to the development of mouse models for experimental myocarditis. It has been shown that pathogenic agents inducing myocarditis in humans can often trigger the disease in mice. Due to multiple etiologies of inflammatory heart diseases in humans, a number of different experimental approaches have been developed to induce myocarditis in mice. Accordingly, experimental myocarditis in mice can be induced by infection with cardiotropic agents, such as coxsackievirus B3 and Edited by: protozoan parasite Trypanosoma cruzi or by activating autoimmune responses against JeanSébastien Silvestre, Institut National de la Santé et de la heart-specific antigens. In certain models, myocarditis is followed by the phenotype of Recherche Médicale dilated cardiomyopathy and the end stage of heart failure. This review describes the most (INSERM), France commonly used mouse models of experimental myocarditis with a focus on the role of Reviewed by: the innate and adaptive immune systems in induction and progression of the disease. Sophie Van Linthout, Charité Medical University of The review discusses also advantages and limitations of individual mouse models in the Berlin, Germany context of the clinical manifestation and the course of the disease in humans. -

Role of Autoimmunity in Dilated Cardiomyopathy Br Heart J: First Published As 10.1136/Hrt.72.6 Suppl.S30 on 1 December 1994
S 30 BrHeartJ 1994;72 (Supplement):S 30-S 34 Role of autoimmunity in dilated cardiomyopathy Br Heart J: first published as 10.1136/hrt.72.6_Suppl.S30 on 1 December 1994. Downloaded from A L P Caforio Persistent viral infection of the myocardium disposition and environmental influences. and autoimmunity are two of the main patho- The genetic predisposition accounts for both genic hypotheses for dilated cardiomyopathy the fact that different autoimmune conditions (DCM). A unifying hypothesis also suggests may be associated in patients or in their family that an initial viral insult may trigger or pre- members and the common finding that single cipitate autoimmunity. Is it becoming clearer autoimmune diseases often run in families. whether DCM is a chronic viral disease, a The inheritance of susceptibility is usually post-infectious autoimmune process, or polygenic. Organ-specific autoimmune dis- genetically determined organ-specific auto- eases are commonly associated with specific immune disease? HLA class II antigens,' but it is not known When tolerance to "self' antigens is lost how the HLA system determines the pre- autoimmune disease results. It is charac- disposition to a specific disease. terised by the presence of circulating autoanti- Most organ-specific autoimmune diseases bodies, which are not necessarily pathogenic are chronic and apparently idiopathic. Organ but represent markers of continuing tissue and disease specific antibodies are found in damage.' In autoimmune disease that is not the affected patients. These antibodies are organ specific there are autoantibodies against also detected in family members, sometimes ubiquitous autoantigens and tissue damage is years before the disease develops, and they generalised. -

Women and Autoimmune Diseases Delisa Fairweather* and Noel R
Women and Autoimmune Diseases DeLisa Fairweather* and Noel R. Rose* Autoimmune diseases affect approximately 8% of the eases tend to cluster in families and in individuals (a per- population, 78% of whom are women. The reasons for the son with one autoimmune disease is more likely to get high prevalence in women are unknown, but circumstantial another), which indicates that common mechanisms are evidence links autoimmune diseases with preceding infec- involved in disease susceptibility. Studies of the preva- tions. Animal models of autoimmune diseases have shown lence of autoimmune disease in monozygotic twins show that infections can induce autoimmune disease. For exam- ple, coxsackievirus B3 (CB3) infection of susceptible mice that genetic as well as environmental factors (such as results in inflammation of the heart (myocarditis) that infection) are necessary for the disease to develop (6). resembles myocarditis in humans. The same disease can Genetic factors are important in the development of be induced by injecting mice with heart proteins mixed with autoimmune disease, since such diseases develop in cer- adjuvant(s), which indicates that an active infection is not tain strains of mice (e.g., systemic lupus erythematosus or necessary for the development of autoimmune disease. lupus in MRL mice) without any apparent infectious envi- We have found that CB3 triggers autoimmune disease in ronmental trigger. However, a body of circumstantial evi- susceptible mice by stimulating elevated levels of proin- dence links diabetes, multiple sclerosis, myocarditis, and flammatory cytokines from mast cells during the innate many other autoimmune diseases with preceding infec- immune response. Sex hormones may further amplify this hyperimmune response to infection in susceptible persons, tions (Table) (7,8). -

Guidelines for Occupational Health Follow up of Communicable Diseases for Manager/Supervisors
Winnipeg Regional Health Authority Occupational and Environmental Safety & Health (OESH) Guidelines for Occupational Health Follow Up of Communicable Diseases For Manager/Supervisors Page | 1 2019.02.04 version 2 Winnipeg Regional Health Authority Occupational and Environmental Safety & Health (OESH) INTRODUCTION ................................................................................................................................................................................. 3 WORKERS COMPENSATION BOARD (WCB) CLAIMS ........................................................................................................................... 5 ANTIBIOTIC RESISTANT ORGANISMS (AROS) ..................................................................................................................................... 6 BLOOD AND BODY FLUID EXPOSURES ............................................................................................................................................... 9 CIMEX LECTULARIUS (BED BUGS) .................................................................................................................................................... 10 CREUTZFELDT-JAKOB DISEASE (CJD) ................................................................................................................................................ 11 DIARRHEA (BACTERIAL, CLOSTRIDIUM DIFFICILE (C. DIFFICILE), VIRAL) ........................................................................................... 12 GROUP A STREPTOCOCCUS ............................................................................................................................................................