209482Orig1s000
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Product Insert (PDF)
PRODUCT INFORMATION Piclamilast Item No. 29170 CAS Registry No.: 144035-83-6 Formal Name: 3-(cyclopentyloxy)-N-(3,5-dichloro- 4-pyridinyl)-4-methoxy-benzamide O Synonyms: RP 73401, RPR 73401 Cl H MF: C18H18Cl2N2O3 FW: 381.3 N O Purity: ≥98% O Supplied as: A solid N Storage: -20°C Cl Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Piclamilast is supplied as a solid. A stock solution may be made by dissolving the piclamilast in the solvent of choice, which should be purged with an inert gas. Piclamilast is soluble in organic solvents such as ethanol and DMSO. The solubility of piclamilast in ethanol is approximately 20 mM and approximately 100 mM in DMSO. Description 1 Piclamilast is a phosphodiesterase 4 (PDE4) inhibitor (IC50 = 0.31 nM). It is selective for PDE4 over PDE1, 2,3 PDE2, PDE3, PDE5, and PDE7A in cell-free assays (IC50s = >100, 40, >100, 14, and >10 μM, respectively). Piclamilast inhibits superoxide production by guinea pig eosinophils and histamine-induced contraction 1,4 in isolated guinea pig trachea (IC50s = 24 and 2 nM, respectively). It inhibits eosinophil, neutrophil, lymphocyte, and TNF-α accumulation in bronchoalveolar lavage fluid (BALF) from ovalbumin-sensitized 5 rats (ED50s = 23.8, 14.1, 19.5, and 14.4 μmol/kg, respectively). Piclamilast also inhibits ovalbumin-induced 2 bronchoconstriction in a guinea pig model of asthma (ED50 = 0.033 mg/kg). References 1. Ashton, M.J., Cook, D.C., Fenton, G., et al. Selective type IV phosphodiesterase inhibitors as antiasthmatic agents. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group R01 (Nasal preparations) Table of Contents Page INTRODUCTION 5 DISCLAIMER 7 GLOSSARY OF TERMS USED IN THIS DOCUMENT 8 ACTIVE SUBSTANCES Cyclopentamine (ATC: R01AA02) 10 Ephedrine (ATC: R01AA03) 11 Phenylephrine (ATC: R01AA04) 14 Oxymetazoline (ATC: R01AA05) 16 Tetryzoline (ATC: R01AA06) 19 Xylometazoline (ATC: R01AA07) 20 Naphazoline (ATC: R01AA08) 23 Tramazoline (ATC: R01AA09) 26 Metizoline (ATC: R01AA10) 29 Tuaminoheptane (ATC: R01AA11) 30 Fenoxazoline (ATC: R01AA12) 31 Tymazoline (ATC: R01AA13) 32 Epinephrine (ATC: R01AA14) 33 Indanazoline (ATC: R01AA15) 34 Phenylephrine (ATC: R01AB01) 35 Naphazoline (ATC: R01AB02) 37 Tetryzoline (ATC: R01AB03) 39 Ephedrine (ATC: R01AB05) 40 Xylometazoline (ATC: R01AB06) 41 Oxymetazoline (ATC: R01AB07) 45 Tuaminoheptane (ATC: R01AB08) 46 Cromoglicic Acid (ATC: R01AC01) 49 2 Levocabastine (ATC: R01AC02) 51 Azelastine (ATC: R01AC03) 53 Antazoline (ATC: R01AC04) 56 Spaglumic Acid (ATC: R01AC05) 57 Thonzylamine (ATC: R01AC06) 58 Nedocromil (ATC: R01AC07) 59 Olopatadine (ATC: R01AC08) 60 Cromoglicic Acid, Combinations (ATC: R01AC51) 61 Beclometasone (ATC: R01AD01) 62 Prednisolone (ATC: R01AD02) 66 Dexamethasone (ATC: R01AD03) 67 Flunisolide (ATC: R01AD04) 68 Budesonide (ATC: R01AD05) 69 Betamethasone (ATC: R01AD06) 72 Tixocortol (ATC: R01AD07) 73 Fluticasone (ATC: R01AD08) 74 Mometasone (ATC: R01AD09) 78 Triamcinolone (ATC: R01AD11) 82 -

Intranasal Rhinitis Agents
Intranasal Rhinitis Agents Therapeutic Class Review (TCR) February 1, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. -

Doxofylline, a Novofylline Inhibits Lung Inflammation Induced By
Pulmonary Pharmacology & Therapeutics 27 (2014) 170e178 Contents lists available at ScienceDirect Pulmonary Pharmacology & Therapeutics journal homepage: www.elsevier.com/locate/ypupt Doxofylline, a novofylline inhibits lung inflammation induced by lipopolysacharide in the mouse Yanira Riffo-Vasquez*, Francis Man, Clive P. Page Sackler Institute of Pulmonary Pharmacology, Institute of Pharmaceutical Science, King’s College London, UK article info abstract Article history: Rational: Doxofylline is a xanthine drug that has been used as a treatment for respiratory diseases for Received 20 December 2013 more than 30 years. In addition to doxofylline being a bronchodilator, some studies have indicated that Received in revised form doxofylline also has anti-inflammatory properties, although little is known about the effect of this drug 30 December 2013 on lung inflammation. Accepted 2 January 2014 Objectives: We have investigated the actions of doxofylline against the effects of Escherichia coli LPS in the lungs of BALB/c mice. Keywords: Methods: Animals have been treated with doxofylline (0.1, 0.3 and 1 mg/kg i.p.) 24, -and 1 h before, and Doxofylline m Neutrophils 6 h after intra-nasal instillation of LPS (10 g/mouse). Readouts were performed 24 h later. fi LPS Results: Doxofylline at 1 and 0.3, but not at 0.1 mg/kg, signi cantly inhibit neutrophil recruitment to the Lung lung induced by LPS (LPS: 208.4 Æ 14.5 versus doxofylline: 1 mg/kg: 106.2 Æ 4.8; 0.3 mg/kg: 4 Inflammation 105.3 Æ 10.7 Â 10 cells/ml). Doxofylline significantly inhibited IL-6 and TNF-a release into BAL fluid in Mice comparison to LPS-treated animals (LPS: 1255.6 Æ 143.9 versus doxofylline 1 mg/kg: 527.7 Æ 182.9; 0.3 mg/kg: 823.2 Æ 102.3 pg/ml). -

Early Protective Effects of Tiotropium Bromide in Patients with Airways Hyperres P O N S I V E N E S S
European Review for Medical and Pharmacological Sciences 2004; 8: 259-264 Early protective effects of tiotropium bromide in patients with airways hyperres p o n s i v e n e s s C. TERZANO, A. PETROIANNI, A. RICCI, L. D’ANTONI, L. ALLEGRA* Department of Cardiovascular and Respiratory Sciences, Respiratory Diseases Unit, Fondazione E. Lorillard Spencer Cenci, “La Sapienza” University - Rome (Italy) *Institute of Respiratory Diseases, University of Milan, Ospedale Maggiore IRCCS – Milan (Italy) Abstract. – Tiotropium is an anticholiner- Introduction gic drug for Ch ronic Obstructive Pulmonary Di sease (COPD) patients, with a peak b ron- Ti o t rop ium is a qu atern a ry amm o niu m chodilator effect observed after 1.5 to 2 hours and a long duration of action. The aim of our co ngener of atropine. It has been developed study was to quantify the early protection of a as a lon g-acting an ticho linergic bro n c h o d i l a- single dose of inhaled tiotropium against metha- tor for inhalation by patients with chronic ob- choline-induced bronchoconstriction in asthmat- s t ructive pu lmon ary d isease (COPD). Dru g ic patients with airway hyperresponsiveness. p ro p e rties o f ant icho lin ergic agent s have Ten subjects (7M, 3F), with history of asthma been well-kno wn to man y cultures for many and a baseline FEV (Forced Expiratory Volume 1 1 1 c e n t u r i e s . In the 1920s the d iscovery o f the sec) >80% of pred icted, were enrolled in the s t u d y. -

Effect of Ipratropium Bromide on Mucociliary Clearance and Pulmonary Function in Reversible Airways Obstruction
Thorax: first published as 10.1136/thx.34.4.501 on 1 August 1979. Downloaded from Thorax, 1979, 34, 501-507 Effect of ipratropium bromide on mucociliary clearance and pulmonary function in reversible airways obstruction DEMETRI PAVIA, J RODERICK M BATEMAN, NOIRIN F SHEAHAN, AND STEWART W CLARKE From the Department of Thoracic Medicine, The Royal Free Hospital, London NW3 2QG, UK ABSTRACT The effects of (a) regular use for one week and (b) a single dose of a synthetic anticholinergic (ipratropium bromide) on lung mucociliary clearance and as a bronchodilator was ascertained in a controlled, double-blind, cross-over study in 12 patients with reversible airways obstruction (mean increase in FEV1 after isoprenaline: 17%, range 10-50%). Two puffs from a metered dose inhaler of either placebo (propellants only) or drug (40 ,ug) were administered four times a day for one week (regular use), and mucociliary clearance was measured, by radioaerosol tracer, at the end of each treatment period and after a control period in which no treatment was given. On the mornings of the measurements after the placebo and drug periods one final dose (single dose) of ipratropium (40 ,ug) or placebo was given 2 5 hours before the start of the test. There was no statistically significant difference between the three mean mucociliary clearance curves (control, placebo, and drug) for the group; however, there was a significantly greater penetration towards the periphery of the lung of the tracer in the test was after drug administration compared with the other two. This increased penetration http://thorax.bmj.com/ attributed to bronchodilatation caused by the drug. -

PDE4-Inhibitors: a Novel, Targeted Therapy for Obstructive Airways Disease Zuzana Diamant, Domenico Spina
PDE4-inhibitors: A novel, targeted therapy for obstructive airways disease Zuzana Diamant, Domenico Spina To cite this version: Zuzana Diamant, Domenico Spina. PDE4-inhibitors: A novel, targeted therapy for obstructive airways disease. Pulmonary Pharmacology & Therapeutics, 2011, 24 (4), pp.353. 10.1016/j.pupt.2010.12.011. hal-00753954 HAL Id: hal-00753954 https://hal.archives-ouvertes.fr/hal-00753954 Submitted on 20 Nov 2012 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Accepted Manuscript Title: PDE4-inhibitors: A novel, targeted therapy for obstructive airways disease Authors: Zuzana Diamant, Domenico Spina PII: S1094-5539(11)00006-X DOI: 10.1016/j.pupt.2010.12.011 Reference: YPUPT 1071 To appear in: Pulmonary Pharmacology & Therapeutics Received Date: 2 October 2010 Revised Date: 5 December 2010 Accepted Date: 24 December 2010 Please cite this article as: Diamant Z, Spina D. PDE4-inhibitors: A novel, targeted therapy for obstructive airways disease, Pulmonary Pharmacology & Therapeutics (2011), doi: 10.1016/j.pupt.2010.12.011 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Therapeutic Class Overview Inhaled Anticholinergics
Therapeutic Class Overview Inhaled Anticholinergics Therapeutic Class Overview/Summary: The inhaled anticholinergics are a class of bronchodilators primarily used in the management of chronic obstructive pulmonary disease (COPD), a condition characterized by progressive airflow restrictions that are not fully reversible.1-3 Symptoms associated with COPD typically include dyspnea, cough, sputum production, wheezing and chest tightness. Specifically, inhaled anticholinergics work via the inhibition of acetylcholine at parasympathetic sites in bronchial smooth muscle causing bronchodilation. Meaningful increases in lung function can be achieved with the use of inhaled anticholinergics in patients with COPD.1-3 The available single-entity inhaled anticholinergics include aclidinium (Tudorza® Pressair), glycopyrrolate (Seebri Neohaler®), ipratropium (Atrovent®, Atrovent® HFA), tiotropium (Spiriva®, Spiriva Respimat®) and umeclidinium (Incruse Ellipta®) with the combination products including glycopyrrolate/indacaterol (Utibron Neohaler®), umeclidinium/vilanterol (Anoro Ellipta®), tiotropium/olodaterol (Stiolto Respimat®) and ipratropium/albuterol, formulated as either an inhaler (Combivent Respimat®) or nebulizer solution (DuoNeb).4-15 Ipratropium, a short-acting bronchodilator, has a duration of action of six to eight hours and requires administration four times daily. Aclidinium, glycopyrrolate, tiotropium and umeclidinium are considered long-acting bronchodilators. Aclidinium is dosed twice daily, while glycopyrrolate, tiotropium and umeclidinium -

COPD Agents Review – October 2020 Page 2 | Proprietary Information
COPD Agents Therapeutic Class Review (TCR) October 1, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. October 2020 -

Preferred Drug List Effective May 1, 2017
Preferred drug list Effective May 1, 2017 Table of Contents 1.0 Analgesics ………………………………………………………………………………………... 1 2.0 Anesthetics ……………………………………………………………………………………….. 1 3.0 Antibiotics and Antivirals ………………………………………………………………………... 1 4.0 Antineoplastics/Immunosuppressants …………………………………………………………… 2 5.0 Cardiovascular Agents …………………………………………………………………………… 2 6.0 Central Nervous System Agents …………………………………………………………………. 3 7.0 Dermatologicals ………………………………………………………………………………….. 4 8.0 Eyes, Ears, Nose, Mouth and Throat …………………………………………………………….. 4 9.0 Endocrine Agents ………………………………………………………………………………… 5 10.0 Gastrointestinal Agents ………………………………………………………………………….. 5 11.0 Blood Modifiers, Nutritionals and Electrolytes ………………………………………………….. 6 12.0 OB/GYN …………………………………………………………………………………………. 6 13.0 Respiratory Agents ………………………………………………………………………………. 7 14.0 Skeletal Muscle Relaxants ……………………………………………………………………….. 8 15.0 Urologicals ……………………………………………………………………………………….. 8 16.0 Immunologicals, Vaccines and Biotechnology Drugs …………………………………………… 8 17.0 Smoking Cessation ………………………………………………………………………………. 8 www.anthem.com/inmedicaid Anthem Blue Cross and Blue Shield is the trade name of Anthem Insurance Companies, Inc., independent licensee of the Blue Cross and Blue Shield Association. ANTHEM is a registered trademark of Anthem Insurance Companies, Inc. The Blue Cross and Blue Shield names and symbols are registered marks of the Blue Cross and Blue Shield Association. Express Scripts, Inc. is a separate company that provides pharmacy services and pharmacy benefit management -
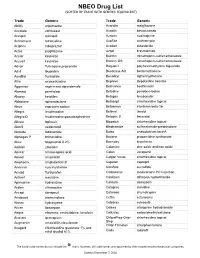
Drug List (SORTED by TRADE with GENERIC EQUIVALENT)
NBEO Drug List (SORTED BY TRADE WITH GENERIC EQUIVALENT) Trade Generic Trade Generic Abilify aripiprazole Avandia rosiglitazone Accolate zafirlukast Avastin bevacizumab Accupril quinapril Azasan azathioprine Achromycin tetracycline AzaSite azithromycin Aciphex rabeprazole Avodart dutasteride Actos pioglitazone Azopt brinzolamide Acular ketorolac Bactrim trimethoprim-sulfamethoxazole Acuvail ketorolac Bactrim DS trimethoprim-sulfamethoxazole Advair fluticasone propionate Baquacil polyhexamethylene biguanide Advil ibuprofen Beconase AQ beclomethasone AeroBid flunisolide Benadryl diphenhydramine Afrin oxymetazoline Bepreve Bepotastine besilate Aggrenox aspirin and dipyridamole Besivance besifloxacin Alamast pemirolast Betadine povidone-iodine Alaway ketotifen Betagan levobunolol Aldactone spironolactone Betasept chlorhexidine topical Aleve naproxen sodium Betaseron interferon beta-1b Allegra fexofenadine Betimol timolol Allegra-D fexofenadine-pseudoephedrine Betoptic S betaxolol Alluvia lopinavir Biopatch chlorhexidine topical Alocril nedocromil Blephamide sulfacetamide-prednisolone Alomide lodoxamide Botox onabotulinum toxinA Alphagan P brimonidine Brolene propamidine isethionate Alrex loteprednol 0.2% Bromday bromfenac Ambien zolpidem Calamine zinc oxide and iron oxide Amicar aminocaproic acid Calan verapamil Amoxil amoxicillin Calgon Vesta chlorhexidine topical Amphocin amphotericin B Capoten captopril Anectine succinylcholine Carafate sucralfate Ansaid flurbiprofen Carbocaine mepivacaine HCl injection Antivert meclizine Cardizem diltiazem