The Statistical Assessment of Nitrate Contamination Status of Ground Water in Balotra Block of District Barmer, Rajasthan, India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RAJASTHAN STATE LEGAL SERVICES AUTHORITY, JAIPUR MEDIATION TRAINING PROGRAMME up to 31-5-2012 S.No
RAJASTHAN STATE LEGAL SERVICES AUTHORITY, JAIPUR MEDIATION TRAINING PROGRAMME UP TO 31-5-2012 S.No. Divisional Head Date of holding the Concerned No. of No. Judicial officer Total No. of No. of No. of Name of trainers Remarks. Quarter Mediation training districts Advocates Trained Trained Referral mediators Judicial Judges. Advocates Offers as Mediator 01 Jaipur HQ 25 .4.2009 to -- 13Adv.+4 02 Dy. 18 02 --- Mr. Prasad Subbanna, 30.4.2009 Adv. Total Sec. RSLSA Advocate and 18 Mediator and co- ordinator, Bangalore. Mr. B.K. Mehta, Advcoate & mediator, Bangalore 02 Jodhpur HQ 31 Marth 2011 to 1st RHC Jodhpur 18 -- 18 -- 25 Mrs. Neena Krishna April,2011 and 9 to Bansal- Home Court 12 April, 2011 Delhi. Shri Arun Kumar Arya- Home Court – Delhi. 03 Jaipur Division 15.7.2011 to Jaipur Distt. 07 08 40+01 42 32 Mr. V.K. Bansal- Home 17.7.2011 Jaipur Metro 11+01 S.W. 14 123 Court,Delhi 22.7.2011 to Dausa 05 04 11 09310384709 24.7.2011 Sikar 04 04 13 Ms. Anju Bajaj 2nd round Jhunjhunu 06 04 12 Chandra- Home 06-01-2012 to 08-1- Alwar 07 08 55 Court,Delhi 2012 and 27-1-2012 09910384712 to 29-1-2012 2nd round 10-2-2012 to 12-2- Anju Bajaj chandana & 2012and 24 to 26-02- V.Khana , Shalinder 2012 JPR DISTT. kaur.(Jaipur Distt.) 11-5-2012 to 13-5- Ms. Neena Krishana 2012 and 25-5-2012 Bansal 09910384633 to 27-5-2012 Sh. Dharmesh Sharma 09910384689 04 Ajmer Division 05.08-2011 to Ajmer 10+01 S.W. -

Balotra Pollution CETP Rajasthan HC.Pdf
- 1 - IN THE HIGH COURT OF JUDICATURE FOR RAJASTHAN AT JODHPUR. O R D E R D.B. Civil Writ Petition No.10986/2015 Balotra Water Pollution Control & Research Foundation Trust (BWPCRT) Versus State of Rajasthan & Ors. Date of Order :: 8th October, 2015 P R E S E N T HON'BLE MR.JUSTICE GOVIND MATHUR HON'BLE MISS JUSTICE JAISHREE THAKUR Mr. M.S.Singhvi, Senior Advocate, assisted by Mr. Vinay Kothari, Mr. Deepak Chandak and Mr. Vineet Dave, for the petitioner. Dr. P.S.Bhati, Additional Advocate General with Mr. S.S.Rathore, for respondent-State. Mr. Digvijay Singh – Respondent in person. Mr. Sanjeet Purohit] for the respondents. .... BY THE COURT : (PER HON'BLE MATHUR,J.) REPORTABLE The Government of Rajasthan constituted a “Jal Pradushan Nivaran Samiti” to construct, operate and maintain Common Effluent Treatment Plant (CETP) in the territorial jurisdiction of Balotra Municipal Board to prevent pollution and to control the effluents, wastes and sewages discharge by Textile Processing Unit situated in the town of Balotra and the surrounding areas. With a view to accelerate the establishment of the CETP and to make provisions for proposed treatment plant, its expansion and carrying out research work relating to pollution control, - 2 - it was found expedient to consolidate all activities under one administrative board, thus, the petitioner trust was established by a registered trust-deed dated 28.9.1995 with following objects :- A. To establish and maintain the CETP for control of water pollution, treatment of industrial waste and discharge -

Before Hon'ble National Green Tribunal, New Delhi in the Matters
Before Hon’ble National Green Tribunal, New Delhi In the Matters of: 1. OA No: 32/2014, Kisan Paryavaran Sangharsh Samiti v/s State of Rajasthan and others, 2. OA No 329/2015, Gram Panchayat Araba v/s State of Rajasthan, and others 3. OA No 34/2014 (THC) Digvijay Singh v/s State of Rajasthan & Ors.) COMMON FINAL REPORT BY THE CHAIRPERSON, NGT APPOINTED COMMITTEE (21stJuly 2021) Hon’ble the National Green Tribunal, New Delhi, constituted three Monitoring Committees vide orders; dated 23/11/2020, 07/12/2020 & another order of the same date 07/12/2020, passed in three matters, namely, 1.O.A No: 32/2014, Kisan Paryavaran Sangharsh Samiti v/s State of Rajasthan and others,2. OA No 329/2015, Gram Panchayat Araba v/s State of Rajasthan, and others, and 3. OA No 34/2014 (THC) Digvijay Singh v/s State of Rajasthan & Ors.). Each Committee had five-member (separate for each application). Composition of the Committees was: - Chairperson, Justice Prakash Tatia, (in all Committees), District Magistrate, Jodhpur, District Magistrate, Pali, District Magistrate, Barmer, (for their respective district). Dr. Ajit Partap Singh, Professor, BITS Pilani, (in all Committees) Shri S. K. Meena, Scientist-D, CPCB, Bhopal, (in all Committees) Shri Amit Sharma, R.O., Jodhpur, Shri R. K. Bora, R.O., Pali, Shri Amit Juyal, R.O. Balotra of SPCB, as per their areas and are the Nodal Officers of the respective Committee. Chairperson of the committees submits following concluding report: - (1) Hon’ble NGT, Principal Bench, New Delhi earlier, pleased to constitute the Special Task Force (STF) for controlling the industrial pollution created by the textile and steel industries in Jodhpur area and 1 textile industries of Balotra, Pali districts. -

List of P.H.E.D. Officers and Their Telephone Nos.(Tentative)
LIST OF P.H.E.D. OFFICERS AND THEIR TELEPHONE NOS.(TENTATIVE) STD TELEPHONE NO. S. NO. NAME OF OFFICER DESIGNATION PLACE CODE OFFICE EXTN. RESI. 2227254 2227114 1 SH. D.B. GUPTA CHIEF SECRETARY JAIPUR 0141 2574477 FAX 2 SH. SANDEEP VERMA PR. SECRETARY JAIPUR 0141 2227464 3 SH. NIRANJAN SAINI PS TO PR. SECY JAIPUR 0141 2227464 4 SMT. ANUPAMA JORWAL JOINT SECRETARY JAIPUR 0141 2385206 3020 5 SH. B.L. MEENA SPL. SECRETARY-IInd JAIPUR 0141 2227184 3022 6 SH.HIRA LAL SAINI ASSTT. SECRETARY JAIPUR 0141 5153222 PBX 3030 7 SH. HARI SHARAN SECTION OFFICER JAIPUR 0141 5153222 PBX 3030 8 SH. DEVKINANDAN SHARMA FA & CAO, RWSSMB JAIPUR 0141 2223093 161 9 SH. BANSIDHAR (Addl. Charge) DY. F.A., RWSSMB JAIPUR 0141 2220423 138 10 SR. A.O. (WORKS) JAIPUR 0141 2225775 150 11 SR. A.O. (IPA), RWSSMB JAIPUR 0141 2221799 137 2221799 12 SH.LALIT KISHORE KAROL ACE & SECY.RWSSMB JAIPUR 0141 2222117 175 SECTION OFFICER 13 SH. RWSSMB JAIPUR 0141 2220904 135 14 SH. D.K. SAINI CE (TECH) & T.M. RWSSMB JAIPUR 0141 2222342 186 SH. KRISHNA LAL AGARWAL 15 9414547803 SE & TA TO CE (TECH.) & TM JAIPUR 0141 197 SH. KSHEMENDRA SHARMA 16 9414943688 E.E. (CMI) JAIPUR 0141 17 SH. BHARAT SINGH 9314145343 E.E.I (T.M. OFFICE) JAIPUR 0141 294 SH. BHUWNESHWAR AGNIHOTRI 18 9928331191 E.E. II (T.M. OFFICE) JAIPUR 0141 197 2703884 19 SH. RAJESH POONIA 9414405490 E.E. III (T.M. OFFICE) JAIPUR 0141 SH. SHRI KRISHAN RUHELA 20 9461305890 E.E. IV (T.M. -

EIA of Balotra Waste Management Project (BWMP), at Kher Village
EIA of Balotra Waste Management Project (BWMP) at Kher Village, Barmer District, Rajasthan Risk assessment and disaster management plan Introduction The principal objective of risk assessment is to identify and quantify the major hazards and risk associated with various operations of the proposed project at Balotra Waste Management facility, which may lead to emergency consequences (disasters) affecting the public health and safety. Based on this information, an emergency preparedness plan has to be prepared to mitigate the consequences. The approach involves hazards identification, hazards assessment, evaluation and developing a Disaster Management Plan (DMP). Risk analysis Risk analysis includes an estimate of the probability or likelihood that an event will occur. Estimation of random incidents totally uncorrected with plant activities may also be taken in to account. Risk can be characterized in qualitative terms as high, medium or low or in quantitative terms using numerical estimates and statistical calculations. Diminishing the likelihood of an accident or minimizing the consequences will reduce overall risk. Evaluating hazards The need for sophisticated techniques for evaluating hazards depends on the result of preliminary hazard analysis. Various techniques for evaluating hazards are as follows: Hazard and Operability Study (HAZOP) Accident Consequence Analysis Event Tree Analysis Fault Tree Analysis Failure Modes, Effects and Criticality Analysis. In order to be in a state of readiness to face the adverse effects of accidents, an Emergency Preparedness Plan (EPP) has to be prepared. Such a plan must cover the possible hazardous situations in the locality and the causes, areas most likely to be affected, on-site and off-site emergency plans, establishment of Emergency Control Centre (ECC), location of emergency services and duties of officers/staff during an emergency. -

Fairs & Festivals, Part VII-B, Vol-XIV, Rajasthan
PRG. 172 B (N) 1,000 CENSUS OF INDIA 1961 VOLUME XIV .RAJASTHAN PART VII-B FAIRS & FESTIVALS c. S. GUPTA OF THE INDIAN ADMINISTRATIVE SERVICE Superintendent of Census Operations, Rajasthan 1966 PREFACE Men are by their nature fond of festivals and as social beings they are also fond of congregating, gathe ring together and celebrating occasions jointly. Festivals thus culminate in fairs. Some fairs and festivals are mythological and are based on ancient traditional stories of gods and goddesses while others commemorate the memories of some illustrious pers<?ns of distinguished bravery or. persons with super-human powers who are now reverenced and idealised and who are mentioned in the folk lore, heroic verses, where their exploits are celebrated and in devotional songs sung in their praise. Fairs and festivals have always. been important parts of our social fabric and culture. While the orthodox celebrates all or most of them the common man usually cares only for the important ones. In the pages that follow an attempt is made to present notes on some selected fairs and festivals which are particularly of local importance and are characteristically Rajasthani in their character and content. Some matter which forms the appendices to this book will be found interesting. Lt. Col. Tod's fascinating account of the festivals of Mewar will take the reader to some one hundred fifty years ago. Reproductions of material printed in the old Gazetteers from time to time give an idea about the celebrations of various fairs and festivals in the erstwhile princely States. Sarva Sbri G. -
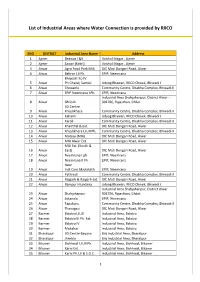
List of Industrial Areas Where Water Connection Is Provided by RIICO
List of Industrial Areas where Water Connection is provided by RIICO SNO DISTRICT Industrial Area Name Address 1 Ajmer Beawar I &II Vaishali Nagar , Ajmer 2 Ajmer Sawar (Kekri) Vaishali Nagar , Ajmer 3 Alwar Agro Food Park MIA DIC Moti Dungari Road, Alwar 4 Alwar Behror I,II Ph. EPIP, Neemrana Bhiwadi I to IV 5 Alwar Ph.Ghatal, Samtal Udyog Bhawan, RIICO Chowk, Bhiwadi I 6 Alwar Chopanki Community Centre, Dhabha Complex, Bhiwadi II 7 Alwar EPIP Neemrana I Ph. EPIP, Neemrana Industrial Area Shahjahanpur, District Alwar - 8 Alwar Ghiloth 301706, Rajasthan, Ghilot IID Centre 9 Alwar Khushkhera Community Centre, Dhabha Complex, Bhiwadi II 10 Alwar Kaharni Udyog Bhawan, RIICO Chowk, Bhiwadi I 11 Alwar Karoli Community Centre, Dhabha Complex, Bhiwadi II 12 Alwar Khairthal & Ext. DIC Moti Dungari Road, Alwar 13 Alwar Khushkhera I,II,III Ph. Community Centre, Dhabha Complex, Bhiwadi II 14 Alwar Matsya (MIA) DIC Moti Dungari Road, Alwar 15 Alwar MIA Alwar Ext. DIC Moti Dungari Road, Alwar MIA Ext. (South & 16 Alwar East) DIC Moti Dungari Road, Alwar 17 Alwar Neemrana I ph. EPIP, Neemrana 18 Alwar Neemrana II Ph. EPIP, Neemrana New 19 Alwar Indl.Com.Majrakath EPIP, Neemrana 20 Alwar Pathredi Community Centre, Dhabha Complex, Bhiwadi II 21 Alwar Rajgarh & Rajgarh Ext. DIC Moti Dungari Road, Alwar 22 Alwar Rampur Mundana Udyog Bhawan, RIICO Chowk, Bhiwadi I Industrial Area Shahjahanpur, District Alwar - 23 Alwar Shahjahanpur 301706, Rajasthan, Ghilot 24 Alwar Sotanala EPIP, Neemrana 25 Alwar Tapukara Community Centre, Dhabha Complex, Bhiwadi II 26 Alwar Thanagazi DIC Moti Dungari Road, Alwar 27 Barmer Balotra I,II,III Industrial Area, Balotra 28 Barmer Balotra III Ph. -

Balotra Maps
# # # # # # # # # # # # # 72°9'30"E 72°10'0"E 72°10'30"E 72°11'0"E 72°11'30"E 72°12'0"E 72°12'30"E 72°13'0"E 72°13'30"E 72°14'0"E 72°14'30"E 72°15'0"E 72°15'30"E 72°16'0"E 72°16'30"E 72°17'0"E 72°17'30"E N " 0 6 KM 3 ' T T 3 5 # ° O O 5 A 2 R D P P A S A P A H H C C C H A 2 P H P 8 O P A N T " A D 0 3 ' 3 D R 5 ° A 5 2 R A A BckyAksrLjkO T R A # N " 0 ' 3 5 ° 5 2 #6 KM N L " 0 ' 3 5 TOWN MAP - 2011 ° 5 D 2 A O uxj ekufp= & 2011 R SS A P A YE B 12 .1 .H # N N N " 0 3 ' 2 5 ° 5 2 NjkA"TVIªhO;N jAkLt HeIkGxHZ@WjAkTY;/S TjAktTEe kHxIZGHWAY #5 KM N " 0 3 ' 2 5 ° 5 2 KATCHA RASTA 8 KM U # dPpk jkLrk TA A TO P B AR BM 105.8 M ER RAILWAY LINE NH 1 jsy ykbZu 12 N " 0 ' 2 5 9 KM ° T 5 O 2 B # AR M KHER )" )" HIGH TENSION ELECTRIC LINE ER JAIRLA mPp {kerk fo|qr ykbZu 7 KM #4 KM # N " 0 ' 2 5 ° 5 #8 KM 2 MuxUjN IiCkIfPyAdL kB OlUheNkDARY #7 KM WATER BODIES:RIVER/PONDS/NALLAH N " 0 tyk'k;@ unh@rkykc ,oa ukyk 3 ' 1 5 ° 5 2 #6 KM 6 KM HILLS N # " 0 3 3 KM ' igkM+h 1 5 ° # 5 2 5 KM # M U N I MUNGRA N C " 0 ' 1 I 5 P ° 5 BM 108.1 A 2 L B U A O 5 KM T U # A N P D O N " T 0 A ' 1 5 4 KM R ° 5 # #2 KM Y 2 #4 KM L 3 KM N " # 0 DAIRY 3 ' 0 5 ° 5 2 U T N HOTEL O GAS AGENCY 3 KM N T I EYE HOSPITAL " IL 0 3 W BM 110.1 # ' A 0 5 R ° A 5 2 EQUALISATION TANK BUS STAND #2KM HOSPITAL #13 KM #1 KM 2 KM Y # R A D PLAYGROUND WATER BOX PARK N U 1 KM N BRICK KILNS " 0 ' R NAGAR PALIKA 1 KM O # 0 SCHOOL PWD 5 ° # 5 11 KM B 2 # L A POST OFFICE P TALKIES KUMS I TRANSPORT OFF. -

Physico-Chemical Analysisfor Different Lakes/River of Udaipur City- a Case Study
International Journal of Scientific & Engineering Research Volume 8, Issue 10, October-2017 612 ISSN 2229-5518 Physico-Chemical Analysisfor Different Lakes/River of Udaipur City- A Case Study 1Nitish Kumar Rai, 2Pawan Chouhan 1(Assistant Professor, Civil Engineering Department, Amity University, Jaipur, Rajasthan) 2 (Junior Environmental Engineer, Pollution Control Board, Balotra, Rajasthan) Abstract: This research paper discusses the water quality parameters for different lakes of Udaipur city. Parameters were analysed during the month of March and April, 2017. For this purpose, five different primary lakes and Ayad river of Udaipur were selected and their physical and chemical water quality parameters were determined. These parameters were determined by normal laboratory methods. For physical water quality analysis, parameters analysed were temperature, colour, odour, turbidity, total solids, total dissolved solids and total suspended solids. And for chemical water quality parameters, pH, alkalinity, hardness, dissolved oxygen, biochemical oxygen demand (BOD) and chemical oxygen demand (COD) were analysed. There were little variations in water quality of lakes. These were dependent upon the location and mixing of water into lakes from different sources. Water qualities of these lakes and river are not up to standards. They need to be treated before their use. Key Words: Water quality parameters, Lakes of Udaipur, Physical water quality, Chemical water quality. —————————— —————————— I. INTRODUCTION Udaipur, formerly the capital of the Mewar Kingdom, is a city in the western Indian state of Rajasthan. It is situated around a series of artificial lakes and is known for its lavish royal residences. Udaipur is also known as the "City of Lakes," the "Venice of the East," or the "Kashmir of Rajasthan". -

Rajasthan List.Pdf
Interview List For Selection Of Appointment Of Notaries In The State Of Rajasthan Date Of Area Of S.No Name Category F.No. Father's Name Address Enrol. No. App'n Practice N- 23/08 Kaveri Path Hari Chand R/638/93 Dt. 1 Sumit Chugh 15.09.15 Jaipur 11013/421/2018 Mansarover Jaipur Chugh 19.12.93 -NC Rajasthan - 302020 N- Seema Madhav Lal B-71, Vijay Singh Pathik R/3514/07 Dt. 2 23.10.15 Bhilwara 11013/422/2018 Kumar Ahir Ahir Nagar Bhilwara Rajasthan 16.12.07 -NC Vpo Rajpura Waya N- Taranagar Dhudhwakhara Tehsil 3 Yashpal Obc 28.07.15 11013/423/2018 Bhag Singh R/ Churu Taranagar Distt.Churu -NC Rajasthan-331029 13/759, Near P.& T. Bharat N- Ramgopal Colony Gurjarwas Ajay R/1025/01 Dt. 4 Kumar Obc 21.09.15 Ajmer 11013/424/2018 Kumawat Nagar Ajmer Rajasthan- 29.07.01 Kumawat -NC 305001 Kamlesh N- Village Post Aspura Tehsil Omprakash R/2684/2003 5 Kumar 01.04.16 Sikar 11013/425/2018 Shrimadhopur Sikar Sharma Dt.30.11.03 Sharma -NC Rajasthan- Dhani Karmari Post N- Ram Naresh Mool Chand Papurana Tehsil Khetri R/1135/2000 6 Obc 31.03.16 Jhunjhunu 11013/426/2018 Singh Saini Saini Jhunjhunu Rajasthan- Dt.17.09.2000 -NC 333503 Paota Tehsil Banka Road Shyam Nagar, N- Kotputli, Prabhoo Vpo-Sangteda Teshil- R/521/2006 7 Arun Kumar Obc 30.03.16 11013/427/2018 Distt. Jaipur Dayal Jat Kotputli-303108 Distt. Dt. 13.05.06 -NC (Raj) Jaipur Rajasthan H.No. 10 Rajasva Colony Chandra N- Gopal Singh Chhoti Sadri, P.O. -

Barmer District
Barmer District Hydrogeological Atlas of Rajasthan Barmer District Contents: List of Plates Title Page No. Plate I Administrative Map 2 Plate II Topography 4 Plate III Rainfall Distribution 4 Plate IV Geological Map 6 Plate V Geomorphological Map 6 Plate VI Aquifer Map 8 Plate VII Stage of Ground Water Development (Block wise) 2011 8 Location of Exploratory and Ground Water Monitoring Plate VIII 10 Stations Depth to Water Level Plate IX 10 (Pre-Monsoon 2010) Water Table Elevation Plate X 12 (Pre-Monsoon 2010) Water Level Fluctuation Plate XI 12 (Pre-Post Monsoon 2010) Electrical Conductivity Distribution Plate XII 14 (Average Pre-Monsoon 2005-09) Chloride Distribution Plate XIII 14 (Average Pre-Monsoon 2005-09) Fluoride Distribution Plate XIV 16 (Average Pre-Monsoon 2005-09) Nitrate Distribution Plate XV 16 (Average Pre-Monsoon 2005-09) Plate XVI Depth to Bedrock 18 Plate XVII Map of Unconfined Aquifer 18 Glossary of terms 19 2013 ADMINISTRATIVE SETUP DISTRICT – BARMER Location: Barmer district is located in the southwestern part of Rajasthan. It is bounded in the North by Jaisalmer district, in the northeast by Jodhpur district, southeast by Jalor district and Pakistan in the West. It stretches between 24⁰ 37' 08.51” to 26⁰ 32' 27.50” North latitude and 70⁰ 04' 02.48’’ to 72⁰ 52' 10.89’’ East longitude covering an approximate area of 28,551 sq kms. The southeastern part of the district is drained by Luni River but significant part of the district does not have a systematic drainage system and is part of Outside basin. Administrative Set-up: Barmer district is administratively divided into eight blocks. -

SPIO of Factories and Boilers Inspection Department
Government of Rajasthan Factories & Boilers Inspection Department List of First Appellate Authority and State Public Information Officer First Appellate officer Jurisdiction State public Information officer Jurisdiction Chief Inspector of Factories & Boilers, Rajasthan, Jaipur Whole Rajasthan Dy. Chief Inspector of Factories & Boilers (HQ), Jaipur Whole Rajasthan Email Id: [email protected] Email Id: [email protected] Postal address: 6-C, Jhalana Doongri Institutional Area, Jaipur-302004 Postal address: 6-C, Jhalana Doongri Institutional Area, Jaipur-302004 List of Assistant Public Information Officer S.No. District State public Information officer SPIO office Email ID. APIO office postal Address. 1 Jaipur Dy. Chief Inspector of Factories & Boilers [email protected] 6-C, Jhalana Doongri Institutional Area, Jaipur -302004 Dausa Jaipur Region Tonk 0141-2709897 2 Kota, Dy. Chief Inspector of Factories & Boilers [email protected] 436, Shastri Nagar, Dadawari Kota 324001 Baran, Kota Jhalawar 0744-2500660 3 Jodhpur & Jaisalmer Dy. Chief Inspector of Factories & Boilers [email protected] Plot No.10 Old police Line Rai ka bag, Jodhpur-342001 Jodhpur 0291-2516693 4 Udaipur & Dy. Chief Inspector of Factories & Boilers [email protected] 62, Basant Vihar, Hiranmagri Sec. 5, Udaipur-313001 Rajsamand Udaipur 0294-2463169 5 Alwar District Except Dy. Chief Inspector of Factories & Boilers [email protected] 209-210 Shanti kunj Alwar-301001 Tijara Tehsil Alwar 0144-2341574