Detection of Citrus Bent Leaf Viroid in Citrus Orchards of Sargodha, Pakistan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chief Minister Self Employment Scheme for Unemployed Educated Youth
Winner List Chief Minister Self Employment Scheme for Unemployed Educated Youth Sargodha Division NIC ApplicantName GuardianName Address WinOrder Bhakkar Bhakkar (Bolan) Key Used: kjhjkbghj 3810106547003 NIAZ HUSSAIN KHUDA BUX MOH MASOOM ABAD BHK TEH&DISTT 1 BHK 3810196198719 M RIZWAN GHOURI M RAMZAN MOH PIR BHAR SHAH NEAE MASJID 2 SADIQ E AKBAR GADOLA 3810122593641 M MUSAWAR HAYAT MALIK MUHAMMAD HAYAT H # 295-B WARD NO 8 CHISHTY 3 HOTLE CHIMNY MOHALLAH 3810106836423 ATIF RANA SARDAR AHMED KHAN NEAR HABIB MASJID BHEAL ROAD 4 BHAKAR 3810102670713 GHULAM MUSTAFA GHULAM HUSSAIN CHAH MUHAMMAD HUSSAIN WALA 5 BHAKKAR 3810192322799 MUHAMMAD IMRAN MUHAMMAD RAMZAN NEAR DHAQ HOSPITAL HOUSE NO 1 6 MOHLAH DOCTORS COLON 3810123681439 MOHAMMAD EJAZ AAMIR MALIK MOHAMMAD AFZAL KHAN CHAK NO 57-58 1M.L SRIOY MUHAJIR 7 TEHS AND DISTT BH 3810126285501 RANA MUDASSAR IQBAL MUHAMMAD IQBAL H NO 134 MOHALLAH SHAHANI NEAR 8 RAILWAY HOSPITAL BH 3810106764713 IFTIKHAR AHMED MUSHTAQ AHMED CHAK NO 48/T.D.A P/O CHAK NO 9 47/T.D.A TEHSIL & DIS 3810106335583 Abdul Rehman Malik Ghulam Hussain Cha Hussain wala Thal R/o Chhima Distt. 10 Bhakkar 3810130519177 ZULFIQAR ALI GHULAM MUHAMMAD KHAN CHAH KHANANWALA P/O KHANSAR 11 TEH & DISTT BHAKKAR 3810105774409 ABDUL RAHIM JEEVAN CHAK NO 203/TDA SRLAY MUHAJIR 12 TEH. & DISTT. BHAKKA 3810126378319 MUHAMMAD ADNAN IQBAL MUHAMMAD IQBAL H NO 3/99 Q TYPE MONDI TOWN 13 BHAKKAR 3620144603149 KHA;LID RAZA MUHAMMAD RAZA CHAK NO 382/WB P.O SAME TEH 14 DUNYA PUR 3810156112899 AFTAB HUSSAIN KHAN FIAZ HUSSAIN KHAN CHAH BAHADUR WALA P/O KHANSAR -

Candidatus Liberibacter Asiaticus’
Page 1 of 41 Razi, Phytopathology 1 1 2 Detection of Citrus Huanglongbing Associated ‘ Candidatus Liberibacter asiaticus’ 3 in Citrus and Diaphorina citri 4 in Pakistan, Seasonal Variability and Implications on Disease Management 5 6 Muhammad F. Razi, Manjunath L. Keremane, Chandrika Ramadugu, Mikeal Roose, 7 Iqrar A. Khan, and Richard F. Lee 8 First and fifth authors: University of Agriculture, Faisalabad, Pakistan; second and sixth authors: 9 U.S. Department of Agriculture-Agricultural Research Service (USDA-ARS), National Clonal 10 Germplasm Repository for Citrus and Dates, Riverside, CA 92507; third and fourth authors: 11 Department of Botany and Plant Sciences, University of California, Riverside, CA 92507 12 Corresponding author: Richard Lee; E-mail address: [email protected] 13 14 ABSTRACT 15 We report the detection of the HLB associated bacterium, ‘ Candidatus Liberibacter asiaticus’ 16 (Las) from both plants and insects in Pakistan and the seasonal variability in the numbers of Las 17 positive psyllid vector, Diaphorina citri . Our studies showed that Las was detectable from trees 18 in areas with maximum temperatures reaching nearly 50°C (average maximum 42°C). However, 19 the bacterium was present at very low levels in psyllids both in summer (June-August) and 20 autumn (September-November) in contrast to reports from Florida, USA, where the bacterium 21 was detectable at very high levels during October-November. We hypothesize that hot summer 22 temperatures in Pakistan may interfere with acquisition and replication of Las in psyllids and Phytopathology "First Look" paper • http://dx.doi.org/10.1094/PHYTO-08-13-0224-R posted 10/17/2013 23 may lead to dead and/or non-transmissible Las in plants. -

Location, Soil and Tree Nutrient Status Influence the Quality of 'Kinnow' Mandarin
INTERNATIONAL JOURNAL OF AGRICULTURE & BIOLOGY ISSN Print: 1560–8530; ISSN Online: 1814–9596 11–098/MFA/2011/13–4–498–504 http://www.fspublishers.org Full Length Article Location, Soil and Tree Nutrient Status Influence the Quality of ‘Kinnow’ Mandarin AHMAD S. KHAN1, MUDASSAR NASEER, AMAN U. MALIK, SHAHZAD M.A. BASRA†, MUHAMMAD S. KHALID, S. KHALID, MUHAMMAD AMIN, BASHARAT A. SALEEM‡, ISHTIAQ A. RAJWANA¶ AND MOEEN UD DIN¶¶ Institute of Horticulture Sciences, University of Agriculture Faisalabad, Pakistan †Department of Crop Physiology, University of Agriculture Faisalabad, Pakistan ‡Hill Fruit Research Station, Sunny Bank, Murree, Pakistan ¶University College of Agriculture, Bahauddin Zakariya University, Multan, Pakistan ¶¶Operations Officer, FAO Country Office, Afghanistan 1Corresponding author’s e-mail: [email protected] ABSTRACT The present study was conducted to determine the relationship of orchard location, soil and tree nutrient status with fruit quality of ‘Kinnow’ mandarin (Citrus reticulata Blanco.) in district Sargodha (major ‘Kinnow’ producing area), Pakistan. The district was divided in four locations comprising of its four tehsils i.e., Bhalwal, Kotmomin, Sargodha and Sillanwali, while from each location seven orchards were randomly selected for soil, leaf and fruit samples collection. Soils from all locations were found slightly alkaline having deficit organic matter and P contents, while optimum in K contents. Foliar N and K contents were within optimum range from Bhalwal and Kotmomin orchards, while foliar P contents were in deficient from all locations. Fruit harvested from Bhalwal exhibited higher juice contents, soluble solids concentration (SSC) and level of ascorbic acid with lowest peel weight and thickness owing to better soils and leaf nutrient contents as compared to other locations. -

Field Appraisal Report Tma Sillanwali
FIELD APPRAISAL REPORT TMA SILLANWALI Prepared by; Punjab Municipal Development Fund Company December-2008 TABLE OF CONTENTS 1. INSTITUTIONAL DEVELOPMENT 1.1 BACKGROUND 2 1.2 METHODOLOGY 2 1.3 DISTRICT PROFILE 2 1.3.1 History 2 1.3.2 Location 2 1.3.3 Area/Demography 2 1.4 TMA/TOWN PROFILE 3 1.4.1 TMA Status 3 1.4.2 Location 3 1.4.3 Area / Demography 3 1.5 TMA STAFF PROFILE 3 1.6 INSTITUTIONAL ASSESSMENT 4 1.6.1 Tehsil Nazim 4 1.6.2 Office of Tehsil Municipal Officer 4 1.7 TEHSIL OFFICER (Planning) OFFICE 8 1.8 TEHSIL OFFICER (Regulation) OFFICE 10 1.9 TEHSIL OFFICER (Finance) OFFICE 11 1.10 TEHSIL OFFICER (Infrastructure & Services) OFFICE 15 2. INFRASTRUCTURE DEVELOPMENT 2.1 ROADS 17 2.2 STREET LIGHTS 17 2.3 WATER SUPPLY 17 2.4 SEWERAGE 18 2.5 SOLID WASTE MANAGEMENT 18 2.6 FIRE FIGHTING 18 2.7 PARKS 19 1 1. INSTITUTIONAL DEVELOPMENT 1.1 BACKGROUND TMA Sillanwali has applied for funding under PMSIP. After initial desk appraisal, PMDFC field team visited the TMA for assessing its institutional and engineering capacity. 1.2 METHODOLOGY Appraisal is based on interviews with TMA staff, open-ended and close-ended questionnaires and agency record. Debriefing sessions and discussions were held with Tehsil Nazim, TMO, TOs and other TMA staff. 1.3 DISTRICT PROFILE 1.3.1 History The district derives its name from the headquarters town of Sargodha, which is a combination of the words „Sar‟ and „Godha‟. Sar, a Hindi word means a water pond while “Godha” was the name of the Hindi Faqir who lived near that pond. -

A Look at the Spatial Inequality in Pakistan:Case Study of District Sargodha
UC Santa Cruz Mapping Global Inequalities Title A look at the Spatial Inequality in Pakistan:Case study of District Sargodha Permalink https://escholarship.org/uc/item/4hd5n6pr Author Ali Asjad Naqvi, Syed Publication Date 2007-12-04 eScholarship.org Powered by the California Digital Library University of California A look at the Spatial Inequality in Pakistan: Case study of District Sargodha Working paper submitted for the conference on Mapping Global Inequalities - Beyond Income Inequality University of California, Santa Cruz Second Draft: 4th December, 2007 Syed Ali Asjad Naqvi1 New School for Social Research 1 Currently enrolled in the first year Ph.D. Economics program at New School for Social Research (New York). A large part of the information was collected while working at Lahore University of Management Sciences (LUMS), Pakistan as a Research Fellow/Data Coordinator in the Economics Department (2005- 2007). Please send comments and feedback at [email protected]) 1 Acknowledgements This paper draws heavily on the research work conducted by Dr. Ali Cheema (Associate Professor and Head of Economics Department, LUMS) and Shandana Khan Mohmand (Teaching Fellow, Social Sciences Department) on the District of Sargodha in the past three years. Dr. Ali Cheema has been a constant source of inspiration throughout my research career at LUMS and has been an integral part for developing the research framework for the overall project. This paper would not have been possible without his support. I would like to acknowledge funding support received from the DRC on the Future State, Institute of Development Studies, University of Sussex; CIDA funded LUMS-McGill Social Enterprise Development Centre; and the Punjab Planning and Development Department for the “LUMS Sargodha Informal Institutions Project”. -

Annual Minimum Temperature Variations in Early 21St Century in Punjab, Pakistan
Journal of Atmospheric and Solar-Terrestrial Physics 137 (2016) 1–9 Contents lists available at ScienceDirect Journal of Atmospheric and Solar-Terrestrial Physics journal homepage: www.elsevier.com/locate/jastp Annual minimum temperature variations in early 21st century in Punjab, Pakistan Misbah Jahangir n, Syeda Maria Ali, Bushra Khalid Department of Environmental Science, International Islamic University Islamabad, Pakistan article info abstract Article history: Climate change is a key emerging threat to the global environment. It imposes long lasting impacts both Received 19 August 2015 at regional and national level. In the recent era, global warming and extreme temperatures have drawn Received in revised form great interest to the scientific community. As in a past century considerable increase in global surface 26 October 2015 temperatures have been observed and predictions revealed that it will continue in the future. In this Accepted 29 October 2015 regard, current study mainly focused on analysis of regional climatic change (annual minimum tem- Available online 30 October 2015 perature trends and its correlation with land surface temperatures in the early 21st century in Punjab) for Keywords: a period of 1979–2013. The projected model data European Centre for Medium-Range Weather Forecasts Minimum temperature (ECMWF) Re-Analysis (ERA-Interim) has been used for eight Tehsils of Punjab i.e., annual minimum Land surface temperature temperatures and annual seasonal temperatures. Trend analysis of annual minimum and annual seasonal Landsat temperature in (Khushab, Noorpur, Sargodha, Bhalwal, Sahiwal, Shahpur, Sillanwali and Chinoit) tehsils Punjab – Pakistan of Punjab was carried out by Regression analysis and Mann Kendall test. Landsat 5 Thematic Mapper (TM) data was used in comparison with Model data for the month of May from the years 2000, 2009 and 2010. -

Mali Sargodha District.Pdf
1 LIST OF CANDIDATES FOR THE POST OF MALI DISTRICT SARGODHA APPLICATI AGE AS ON Mark Mark Total NEW SR. NO. FORM NO. NAME FATHER NAME GENDER RELEG: CNIC NO. DOB 7 Apr 2021 ADDRESS DOMICILE DISTRICT EDU: MOB NO QUOTA EXPERIENCE DOCUMENT STATUS REMARKS Merit ON ID NO. Y M D Obtain Obtain Marks 1 1 98 M. Riaz Male Islam 38403-8185100-5 9-Apr-01 19 11 28 Faisal Town, Streeet No. 4 Sargodha Sargodha Matric Open Nil REJECTED Affidavit & Experience Not ed in ed in M. Zeeshan 0301-1478924 Attached Test Intervi 2 2 49 M. Riaz Male Islam 38403-9309144-3 26-Sep-98 22 6 11 Chak No. 53, Janoobi Sargodha Sargodha Matric Open Nil REJECTED Affidavit & Experience Not Mohsin Riaz ew 0343-8012791 Attached 3 3 50 M. Ramzan Male Islam 38402-0120365-1 28-Feb-93 28 1 9 Asar Sarkharo, P.O Dharwal, Sargodha Sargodha Matric Open Nil REJECTED Affidavit & Experience Not M. Shakeel 0304-9120648 Attached 4 4 61 Akram Masih Male Christian 38403-5606473-5 10-Jan-00 21 2 27 Chak No. 43, Giyanwala P.O Sargodha Sargodha Middle Open Nil REJECTED Affidavit & Experience Not Shehroon Masih Nil Attached 5 5 80 Nasir Hameed Male Islam 38403-7925256-1 25-Aug-98 22 7 12 House No. 9, P.T.S Sargodha Sargodha Middle Open Nil REJECTED Affidavit & Experience Not Sheraz Baloch 0308-1209491 Attached 6 6 104 M.aslam Male Islam 38403-7222978-5 22-Oct-00 20 5 15 Johar Colony House no. 2 Mohallah Sargodha Matric Open Nil REJECTED Affidavit & Experience Not M. -
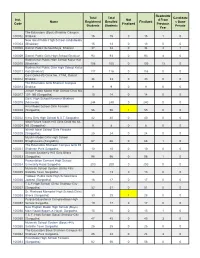
Inst. Code Name Total Registered Students Total Enrolled Students
Readmitte Total Total Candidate Inst. Not d From Name Registered Enrolled Finalized s Gone Code Finalized Previous Students Students Private Year The Educators (Boys) Bhakkar Campus 100002 Bhakkar 15 15 0 15 1 0 New Ideal Public High School Jandanwala 100003 (Bhakkar) 15 13 0 13 0 0 100008 District Public School Boys, Bhakkar 37 34 0 34 3 1 100009 District Public Girls High School Bhakkar 93 93 0 93 4 0 Roshan Kal Public High School Kallur Kot 100010 (Bhakkar) 108 105 0 105 13 0 Roshan Kal Public Girls High School Kallur 100011 Kot (Bhakkar) 117 116 0 116 0 0 Govt Girls H/S Chak No. 71/ML District 100012 Bhakkar. 34 33 0 33 0 0 The Educators Girls Bhakkar Campus 100014 Bhakkar 9 9 0 9 0 0 Jinnah Public Model High School Chak No 100017 107- NB (Sargodha) 15 14 0 14 0 0 Govt. High School Kammar Mashani 100019 (Mianwali) 244 240 0 240 0 0 Hira Model School Girls Farooka 100020 (Sargodha) 56 56 1 55 0 0 100022 Kims Girls High School N.S.T Sargodha. 42 40 0 40 0 0 Ideal Future Vision H/S Girls Chak No 84- 100024 NB (Sargodha) 9 8 0 8 0 0 Islamic Ideal School Girls Farooka 100029 (Sargodha). 25 24 0 24 0 0 Muslim Model Girls High School 100030 Bhagtanwala (Sargodha) 67 66 0 66 1 0 The Educators Shaheen Campus Girls 93 100031 Shaheen Park Sargodha. 10 10 0 10 0 0 Science Academy H/S Girls Bhera 100033 (Sargodha). -

Grand Total Bhalwal Bhera Kot Momin Sahiwal
TEHSIL WISE SUMMARY OF RATIONALIZATION DISTRICT SARGODHA Tehsil DEO (M) DEO (SE) DEO (W) Grand Total Bhalwal 1 17 12 30 Bhera 1 5 4 10 Kot Momin 9 8 17 Sahiwal 4 10 6 20 Sargodha 14 42 13 69 Shahpur 5 7 6 18 Sillanwali 1 13 3 17 Grand Total 35 102 44 181 Sr. # NAME CONTACT # Wing Tehsil FROM SCHOOL TO SCHOOL DESIGNATION DEO (W) Bhera 38470732 - GGES KHALID ENGLISH MEDIUM BHERA 38470725 - GGPS MC NO.2 BHERA PST (Arts) - 14 3074863281 (غزالہ یاسمین) Ghazala Yasmeen 1 DEO (SE) Sargodha 38420662 - GGHS MC BLOCK NO.14 SARGODHA 38420631 - GGES MUHAMMAD ABAD (MUHAMMADI COLONY) SGD PST (Arts) - 14 3037687251 (فہمیدہ بیگم) Fehmeeda Begum 2 DEO (W) Bhalwal 38410531 - GGES CHAK NO. 7 SB SYEDAN WALA 38410528 - GMPS CHAK NO.9 NB LOKRI PST (Arts) - 14 3043700779 (نرگس سلطانہ) Nargis Sultana 3 DEO (W) Bhalwal 38410522 - GGES CHAK NO.14 NB 38410510 - GGES CHAK NO.11 ML PST (Science/Maths) - 14 3046068081 (انمول حنا) Anmol Hina 4 DEO (SE) Sargodha 38420427 - GHS CHAK NO.52 A-NB 38420403 - GPS MC BLOCK NO.23-A SARGODHA PST - 14 3458627649 (هللا دتہ) Allah Ditta 5 DEO (SE) Sargodha 38420385 - GHS MITHA LAK 38420305 - GPS CHAK NO.76 SB PST (Arts) - 14 3450756592 (چوہدری دمحم الطاف) M Altaf 6 DEO (W) Shahpur 38430409 - GGES NO.1 JHAWARIAN 38430395 - GGPS BHABHRANI PST (Arts) - 14 3041045232 (زاہدہ پروین) Zahida Perveen 7 DEO (SE) Shahpur 38430016 - GHS JALPANA SHAHPUR 38420440 - GPS CHAK NO.4 RAKH DHAREMA PST (Arts) - 14 3018899609 (دمحم نواز) Muhammad Nawaz 8 9 Muhammad Sarfraz 3336790967 DEO (M) Shahpur 38430168 - GPS HUKAM PUR 38430196 - GPS SULTAN PUR -
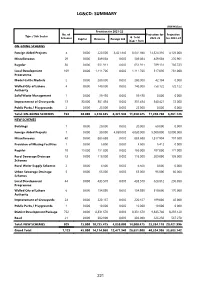
Lg&Cd: Summary
LG&CD: SUMMARY (PKR Million) Provision for 2021-22 No. of Projection for Projection Type / Sub Sector G. Total Schemes Capital Revenue Foreign Aid 2022-23 for 2023-24 (Cap + Rev) ON-GOING SCHEMES Foreign Aided Projects 4 0.000 220.000 8,421.940 8,641.940 14,324.310 4,129.000 Miscellaneous 29 0.000 589.084 0.000 589.084 469.604 270.901 Regular 30 0.000 651.911 0.000 651.911 789.131 786.723 Local Development 109 0.000 1,111.706 0.000 1,111.706 517.096 281.000 Programme Model Cattle Markets 2 0.000 280.000 0.000 280.000 42.104 0.000 Walled City of Lahore 4 0.000 140.000 0.000 140.000 758.122 722.122 Authority Solid Waste Management 1 0.000 59.430 0.000 59.430 0.000 0.000 Improvement of Graveyards 13 30.000 301.454 0.000 331.454 340.421 72.000 Public Parks / Playgrounds 2 0.000 25.000 0.000 25.000 0.000 0.000 Total: ON-GOING SCHEMES 194 30.000 3,378.585 8,421.940 11,830.525 17,240.788 6,261.746 NEW SCHEMES Buildings 1 0.000 20.000 0.000 20.000 60.000 0.000 Foreign Aided Projects 1 0.000 30.000 4,050.000 4,080.000 9,000.000 19,000.000 Miscellaneous 40 0.000 885.688 0.000 885.688 1,817.904 797.500 Provision of Missing Facilities 1 0.000 5.000 0.000 5.000 5.412 0.000 Regular 18 15.000 151.000 0.000 166.000 287.000 121.000 Rural Sewerage Drainage 13 0.000 118.000 0.000 118.000 209.000 105.000 Schemes Rural Water Supply Schemes 2 0.000 6.500 0.000 6.500 0.000 0.000 Urban Sewerage Drainage 5 0.000 65.000 0.000 65.000 95.000 60.000 Schemes Local Development 44 0.000 435.570 0.000 435.570 628.812 296.098 Programme Walled City of Lahore 6 0.000 154.880 0.000 154.880 510.000 170.000 Authority Improvement of Graveyards 24 0.000 220.167 0.000 220.167 189.000 46.000 Public Parks / Playgrounds 1 0.000 10.000 0.000 10.000 10.000 0.000 District Development Package 752 0.000 8,331.670 0.000 8,331.670 9,845.740 8,259.128 Road 21 0.000 302.000 0.000 302.000 626.250 567.270 Total: NEW SCHEMES 929 15.000 10,735.475 4,050.000 14,800.475 23,284.118 29,421.996 Grand Total 1,123 45.000 14,114.060 12,471.940 26,631.000 40,524.906 35,683.742 221 LG&CD (PKR Million) Accum. -
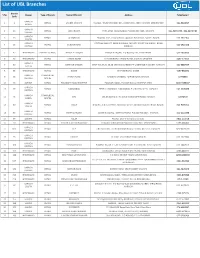
To Download UBL Branch List
List of UBL Branches Branch S No Region Type of Branch Name Of Branch Address Telephone # Code KARACHI 1 2 RETAIL LANDHI KARACHI H-G/9-D, TRUST CERAMIC IND., LANDHI IND. AREA KARACHI (EPZ) EXPORT 021-5018697 NORTH KARACHI 2 19 RETAIL JODIA BAZAR PARA LANE, JODIA BAZAR, P.O.BOX NO.4627, KARACHI. 021-32434679 , 021-32439484 CENTRAL KARACHI 3 23 RETAIL AL-HAROON Shop No. 39/1, Ground Floor, Opposite BVS School, Sadder, Karachi 021-2727106 SOUTH KARACHI CENTRAL BANK OF INDIA BUILDING, OPP CITY COURT,MA JINNAH ROAD 4 25 RETAIL BUNDER ROAD 021-2623128 CENTRAL KARACHI. 5 47 HYDERABAD AMEEN - ISLAMIC PRINCE ALLY ROAD PRINCE ALI ROAD, P.O.BOX NO.131, HYDERABAD. 022-2633606 6 46 HYDERABAD RETAIL TANDO ADAM STATION ROAD TANDO ADAM, DISTRICT SANGHAR. 0235-574313 KARACHI 7 52 RETAIL DEFENCE GARDEN SHOP NO.29,30, 35,36 DEFENCE GARDEN PH-1 DEFENCE H.SOCIETY KARACHI 021-5888434 SOUTH 8 55 HYDERABAD RETAIL BADIN STATION ROAD, BADIN. 0297-861871 KARACHI COMMERCIAL 9 65 NAPIER ROAD KASSIM CHAMBERS, NAPIER ROAD,KARACHI. 32775993 CENTRAL CENTRE 10 66 SUKKUR RETAIL FOUJDARY ROAD KHAIRPUR FOAJDARI ROAD, P.O.BOX NO.14, KHAIRPUR MIRS. 0243-9280047 KARACHI 11 69 RETAIL NAZIMABAD FIRST CHOWRANGI, NAZIMABAD, P.O.BOX NO.2135, KARACHI. 021-6608288 CENTRAL KARACHI COMMERCIAL 12 71 SITE UBL BUILDING S.I.T.E.AREA MANGHOPIR ROAD, KARACHI 32570719 NORTH CENTRE KARACHI 13 80 RETAIL VAULT Shop No. 2, Ground Floor, Nonwhite Center Abdullah Harpoon Road, Karachi. 021-9205312 SOUTH KARACHI 14 85 RETAIL MARRIOT ROAD GILANI BUILDING, MARRIOT ROAD, P.O.BOX NO.5037, KARACHI. -

Subjects 92.14% 86.67% PASS
31/03/2018 WWW.SEDiNFO.NET Punjab Examination Commission Gazette 2018 - Grade 8 District SARGODHA CRITERIA FOR RESULT OF GRADE 8 Criteria SARGODHA Punjab Status Minimum 33% marks in WWW.StudyNowPK.COMall subjects 92.14% 86.67% PASS Pass + Pass Pass + Minimum 33% marks in four subjects and 28 to 32 92.49% 88.68% with Grace marks in one subject Marks Pass + Pass with Grace Pass + Pass with grace marks + Minimum 33% marks in four 98.90% 96.47% Marks + subjects and 10 to 27 marks in one subject Promoted to Next Class Candidate scoring minimum 33% marks in all subjects will be considered "Pass" One star (*) on total marks indicates that the candidate has passed with grace marks. Two stars (**) on total marks indicate that the candidate is promoted to next class. http://osrs.punjab.gov.pk/ WWW.StudyNowPK.COM 1/332 31/03/2018 WWW.SEDiNFO.NET Punjab Examination Commission Gazette 2018 - Grade 8 PUNJAB EXAMINATION COMMISSION, RESULT INFORMATION GRADE 8 EXAMINATION, 2018 DISTRICT: SARGODHA Students Students Students Pass % with Pass + Promoted Pass + Gender WWW.StudyNowPK.COMRegistered Appeared Pass 33% marks Students Promoted % Male 16188 15979 14995 93.84 15849 99.19 Public School Female 15467 15241 14174 93.00 15144 99.36 Male 4588 4148 3669 88.45 4060 97.88 Private School Female 4395 4110 3720 90.51 4064 98.88 Male 1213 1097 901 82.13 1026 93.53 Private Candidate Female 682 615 495 80.49 595 96.75 42533 41190 37954 http://osrs.punjab.gov.pk/ WWW.StudyNowPK.COM 2/332 31/03/2018 WWW.SEDiNFO.NET Punjab Examination Commission Gazette 2018 - Grade