TROGARZO™ (Ibalizumab-Uiyk)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1: Clinical Pharmacokinetics 1
1: CLINICAL PHARMACOKINETICS 1 General overview: clinical pharmacokinetics, 2 Pharmacokinetics, 4 Drug clearance (CL), 6 Volume of distribution (Vd), 8 The half-life (t½), 10 Oral availability (F), 12 Protein binding (PB), 14 pH and pharmacokinetics, 16 1 Clinical pharmacokinetics General overview General overview: clinical pharmacokinetics 1 The ultimate aim of drug therapy is to achieve effi cacy without toxicity. This involves achieving a plasma concentration (Cp) within the ‘therapeutic window’, i.e. above the min- imal effective concentration (MEC), but below the minimal toxic concentration (MTC). Clinical pharmacokinetics is about all the factors that determine variability in the Cp and its time-course. The various factors are dealt with in subsequent chapters. Ideal therapeutics: effi cacy without toxicity Minimum Toxic Concentration (MTC) Ideal dosing Minimum Effective Concentration (MEC) Drug concentration Time The graph shows a continuous IV infusion at steady state, where the dose-rate is exactly appropriate for the patient’s clearance (CL). Inappropriate dosing Dosing too high in relation to the patient’s CL – toxicity likely Minimum Toxic Concentration (MTC) Minimum Effective Concentration (MEC) Dosing too low in relation to the Drug concentration patient’s CL – drug may be ineffective Time Some reasons for variation in CL Low CL High CL Normal variation Normal variation Renal impairment Increased renal blood fl ow Genetic poor metabolism Genetic hypermetabolism Liver impairment Enzyme induction Enzyme inhibition Old age/neonate 2 General overview Clinical Pharmacokinetics Pharmacokinetic factors determining ideal therapeutics If immediate effect is needed, a loading dose (LD) must be given to achieve a desired 1 concentration. The LD is determined by the volume of distribution (Vd). -

Pros and Cons of Entry and Fusion Inhibitors (Review)
MOLECULAR MEDICINE REPORTS 19: 1987-1995, 2019 Investigational drugs in HIV: Pros and cons of entry and fusion inhibitors (Review) EMMANUELE VENANZI RULLO1,2, MANUELA CECCARELLI1, FABRIZIO CONDORELLI3, ALESSIO FACCIOLÀ1, GIUSEPPA VISALLI4, FRANCESCO D'ALEO1, IVANA PAOLUCCI1, BRUNO CACOPARDO5, MARILIA RITA PINZONE2-5, MICHELE DI ROSA6, GIUSEPPE NUNNARI1 and GIOVANNI F. PELLICANÒ7 1Department of Clinical and Experimental Medicine, Unit of Infectious Diseases, University of Messina, I-90124 Messina, Italy; 2Department of Pathology and Laboratory Medicine, School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; 3Department of Pharmacological Sciences, University of Eastern Piedmont ‘A. Avogadro’, I-13100 Novara; 4Department of Biomedical and Dental Sciences and Morphofunctional Imaging, University of Messina, I-90124 Messina; 5Department of Clinical and Experimental Medicine, University of Catania, I-95123 Catania; 6Department of Biomedical and Biotechnological Sciences, Human Anatomy and Histology Section, University of Catania, I-95123 Catania; 7Department of Human Pathology of the Adult and the Developmental Age ‘G. Barresi’, Unit of Infectious Diseases, University of Messina, I-98122 Messina, Italy Received September 2, 2018; Accepted November 29, 2018 DOI: 10.3892/mmr.2019.9840 Abstract. Despite the profound changes and improve- 4. Gp41 antagonists ments reached in the field of HIV treatment, tolerability and 5. CD4 antagonists adherence to highly active antiretroviral therapy remains a 6. Discussion challenge. Furthermore, multi-experienced patients could take advantage of drugs with different mechanisms of action to combat the spread of resistance to actual therapy. For these 1. Introduction reasons identification of new HIV drugs is crucial. Among all the molecules that at present are under investigation, entry HIV continues to be an important challenge and a major and fusion inhibitors pose an interesting class owing to their global public health issue. -

Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients
antibodies Review Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients Andrew T. Lucas 1,2,3,*, Ryan Robinson 3, Allison N. Schorzman 2, Joseph A. Piscitelli 1, Juan F. Razo 1 and William C. Zamboni 1,2,3 1 University of North Carolina (UNC), Eshelman School of Pharmacy, Chapel Hill, NC 27599, USA; [email protected] (J.A.P.); [email protected] (J.F.R.); [email protected] (W.C.Z.) 2 Division of Pharmacotherapy and Experimental Therapeutics, UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] 3 Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-919-966-5242; Fax: +1-919-966-5863 Received: 30 November 2018; Accepted: 22 December 2018; Published: 1 January 2019 Abstract: The rapid advancement in the development of therapeutic proteins, including monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs), has created a novel mechanism to selectively deliver highly potent cytotoxic agents in the treatment of cancer. These agents provide numerous benefits compared to traditional small molecule drugs, though their clinical use still requires optimization. The pharmacology of mAbs/ADCs is complex and because ADCs are comprised of multiple components, individual agent characteristics and patient variables can affect their disposition. To further improve the clinical use and rational development of these agents, it is imperative to comprehend the complex mechanisms employed by antibody-based agents in traversing numerous biological barriers and how agent/patient factors affect tumor delivery, toxicities, efficacy, and ultimately, biodistribution. -

Keeping up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A
Keeping Up with FDA Drug Approvals: 60 New Drugs in 60 Minutes Elizabeth A. Shlom, PharmD, BCPS Senior Vice President & Director Clinical Pharmacy Program | Acurity, Inc. Privileged and Confidential April 10, 2019 Privileged and Confidential Program Objectives By the end of the presentation, the pharmacist or pharmacy technician participant will be able to: ▪ Identify orphan drugs and first-in-class medications approved by the FDA in 2018. ▪ List five new drugs and their indications. ▪ Identify the place in therapy for three novel monoclonal antibodies. ▪ Discuss at least two new medications that address public health concerns. Dr. Shlom does not have any conflicts of interest in regard to this presentation. Both trade names and generic names will be discussed throughout the presentation Privileged and Confidential 2018 NDA Approvals (NMEs/BLAs) ▪ Lutathera (lutetium Lu 177 dotatate) ▪ Braftovi (encorafenib) ▪ Vizimpro (dacomitinib) ▪ Biktarvy (bictegravir, emtricitabine, ▪ TPOXX (tecovirimat) ▪ Libtayo (cemiplimab-rwic) tenofovir, ▪ Tibsovo (ivosidenib) ▪ Seysara (sarecycline) alafenamide) ▪ Krintafel (tafenoquine) ▪ Nuzyra (omadacycline) ▪ Symdeko (tezacaftor, ivacaftor) ▪ Orilissa (elagolix sodium) ▪ Revcovi (elapegademase-lvir) ▪ Erleada (apalutamide) ▪ Omegaven (fish oil triglycerides) ▪ Tegsedi (inotersen) ▪ Trogarzo (ibalizumab-uiyk) ▪ Mulpleta (lusutrombopag) ▪ Talzenna (talazoparib) ▪ Ilumya (tildrakizumab-asmn) ▪ Poteligeo (mogamulizumab-kpkc) ▪ Xofluza (baloxavir marboxil) ▪ Tavalisse (fostamatinib disodium) ▪ Onpattro (patisiran) -

CPIC Guideline for Pharmacogenetics-Guided Warfarin Dosing – Supplement V2.0 1 Table of Contents Guideline Updates
Supplement to: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for Pharmacogenetics-guided Warfarin Dosing: 2016 Update Julie A. Johnson1, Kelly Caudle2, Li Gong3, Michelle Whirl-Carrillo3, C. Michael Stein4, Stuart A. Scott5, Ming Ta Michael Lee6 , Brian F. Gage7, Stephen E. Kimmel8,9, Minoli A. Perera10, Jeffrey L. Anderson11, Munir Pirmohamed12, Teri E. Klein3, Nita A. Limdi13, Larisa H. Cavallari1, Mia Wadelius14 1Department of Pharmacotherapy and Translational Research, College of Pharmacy, and Center for Pharmacogenomics, University of Florida, Gainesville, Florida, USA 2Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, TN 3Department of Genetics, Stanford University, Stanford, California, USA 4Division of Clinical Pharmacology Vanderbilt Medical School, Nashville, TN, USA 5Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA 6Laboratory for International Alliance on Genomic Research, RIKEN Center for Integrative Medical Sciences, Yokohama, Japan; National Center for Genome Medicine; Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan; Genomic Medicine Institute Geisinger Health system, Danville, PA 7Department of Internal Medicine, Washington University in St. Louis, St. Louis, Missouri 8Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA 9Department of Medicine and Department of Biostatistics and Epidemiology, University of Pennsylvania -

An HIV Clinician's View
HIV Meets Oncology: An HIV Clinician’s View Merceditas S. Villanueva MD Director, HIV/AIDS Program Yale University School of Medicine HIV and Cancer Retreat April 30, 2019 Financial Disclosures • None Case Presentation • 58 year old man diagnosed with HIV in 1996 • Early years: serial ART mono and dual therapy with multidrug resistance • Moved to CT 2005-worked in pharmaceutical field (ART development) • I met him in 2009-applying for disability ART Over Time Saquinavir Tenofovir Emtricitabine Stavudine Ritonavir Atazanavir Maraviroc Elvitegravir Zidovudine Doravirine Indinavir Enfuvirtide Raltegravir Nelfinavir Ibalizumab 1985 1990 1995 2000 2005 2010 2015 2018 Didanosine Tenofovir Lamivudine Efavirenz Darunavir Rilpivirine alafenamide Abacavir Fosemprenavir Dolutegravir Nevirapine Lopinavir+Ritonavir Etravirine ART in Single Tablet Regimen Now 1 pill a Day 2-3 ARVs co-formulated ART Simpler and Safer AN AIDS TIMELINE: The 1st Decade RONALD REAGAN GEORGE H.W. BUSH 1981-1989 1989-1993 ’85 ’87 AZT ’82 First approved HIV “AIDS” ’83 Virus test isolation 1981 AN AIDS TIMELINE: The 2nd Decade GEORGE H.W. BUSH WILLIAM CLINTON 1989-1993 1993-2000 92’ ddl/ddC 93’ d4t Dual NRTI’s HAART ’97: NEL, DLV, Combivir ’95: Saquinavir ’96: NVP, IDV, RIT ’92 AIDS ’96 HIV leading cause viral load of death approved adults 25-44 yrs old AIDS Deaths Red down Ribbon 40% due Tony to Awards HAART 19901 1994 1995 ‘96 My Pt was diagnosed HIV+ AN AIDS TIMELINE: THE 3rd Decade … GEORGE W. BUSH BARACK H. OBAMA 2001-2008 2009 - 2016 ’00 ’01 ’03 ‘04 ’05 ‘06 Darunavir ’07 ’08 Trizivir Tenofo Fuzeon Truvada Tipranavir Maraviroc Etravirine -Atripla vir Raltegravir Lopinavir ’00 13th Int’l AIDS : Conf in PEPFAR 25 30 Durban SA $15B/5 yrs years years 2000 AN AIDS TIMELINE: THE 4th Decade … DONALD J. -

Efavirenz) Capsules and Tablets 3 Rx Only
1 SUSTIVA® 2 (efavirenz) capsules and tablets 3 Rx only 4 DESCRIPTION 5 SUSTIVA® (efavirenz) is a human immunodeficiency virus type 1 (HIV-1) specific, non- 6 nucleoside, reverse transcriptase inhibitor (NNRTI). 7 Capsules: SUSTIVA is available as capsules for oral administration containing either 8 50 mg, 100 mg, or 200 mg of efavirenz and the following inactive ingredients: lactose 9 monohydrate, magnesium stearate, sodium lauryl sulfate, and sodium starch glycolate. 10 The capsule shell contains the following inactive ingredients and dyes: gelatin, sodium 11 lauryl sulfate, titanium dioxide, and/or yellow iron oxide. The capsule shells may also 12 contain silicon dioxide. The capsules are printed with ink containing carmine 40 blue, 13 FD&C Blue No. 2, and titanium dioxide. 14 Tablets: SUSTIVA is available as film-coated tablets for oral administration containing 15 600 mg of efavirenz and the following inactive ingredients: croscarmellose sodium, 16 hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline 17 cellulose, and sodium lauryl sulfate. The film coating contains Opadry® Yellow and 18 Opadry® Clear. The tablets are polished with carnauba wax and printed with purple ink, 19 Opacode® WB. 20 Efavirenz is chemically described as (S)-6-chloro-4-(cyclopropylethynyl)-1,4-dihydro-4- 21 (trifluoromethyl)-2H-3,1-benzoxazin-2-one. 22 Its empirical formula is C14H9ClF3NO2 and its structural formula is: 1 of 45 Approved v2.0 F C 3 Cl O NO 23 H 24 Efavirenz is a white to slightly pink crystalline powder with a molecular mass of 315.68. 25 It is practically insoluble in water (<10 µg/mL). -

TROGARZO (Ibalizumab-Uiyk) RATIONALE for INCLUSION IN
TROGARZO (ibalizumab-uiyk) RATIONALE FOR INCLUSION IN PA PROGRAM Background Trogarzo (ibalizumab-uiyk) is a recombinant humanized monoclonal antibody that blocks HIV-1 from infecting CD4+ T-cells. This medication blocks the HIV-1 virus from entering the host cell by interfering with post-attachment steps required for the entry of HIV-1 virus that occurs via cell fusion. The binding specificity of ibalizumab-uiyk to domain 2 of CD4 allows ibalizumab-uiyk to block viral entry into host cells without causing immunosuppression (1). Regulatory Status FDA approved indication: Trogarzo, a CD4-directed post-attachment HIV-1 inhibitor, in combination with other antiretroviral(s), is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily treatment-experienced adults with multidrug resistant HIV-1 infection failing their current antiretroviral regimen (1). Immune reconstitution inflammatory syndrome has been reported in one patient treated with Trogarzo in combination with other antiretrovirals. During the initial phase of combination antiretroviral therapies, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections, which may necessitate further evaluation and treatment(1). Phenotypic and genotypic test results revealed no evidence of cross-resistance between ibalizumab-uiyk and any of the approved classes of anti-retroviral drugs (CCR5 co-receptor antagonists, gp41 fusion inhibitors, integrase strand transfer inhibitors [INSTIs], non-nucleos(t)ide reverse transcriptase inhibitors [NNRTIs], nucleos(t)ide reverse transcriptase inhibitors [NRTIs], or protease inhibitors [PIs]). Ibalizumab-uiyk is active against HIV-1 resistant to all approved antiretroviral agents and exhibits antiretroviral activity against R5-tropic, X4-tropic, and dual-tropic HIV-1 (1). -
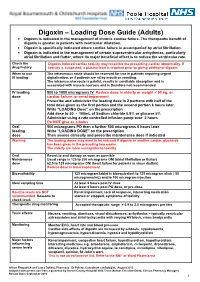
Digoxin – Loading Dose Guide (Adults) Digoxin Is Indicated in the Management of Chronic Cardiac Failure
Digoxin – Loading Dose Guide (Adults) Digoxin is indicated in the management of chronic cardiac failure. The therapeutic benefit of digoxin is greater in patients with ventricular dilatation. Digoxin is specifically indicated where cardiac failure is accompanied by atrial fibrillation. Digoxin is indicated in the management of certain supraventricular arrhythmias, particularly atrial fibrillation and flutter, where its major beneficial effect is to reduce the ventricular rate. Check the Digoxin-induced cardiac toxicity may resemble the presenting cardiac abnormality. If drug history toxicity is suspected, a plasma level is required prior to giving additional digoxin. When to use The intravenous route should be reserved for use in patients requiring urgent IV loading digitalisation, or if patients are nil by mouth or vomiting. The intramuscular route is painful, results in unreliable absorption and is associated with muscle necrosis and is therefore not recommended. IV loading 500 to 1000 micrograms IV Reduce dose in elderly or weight < 50 kg, or dose cardiac failure, or renal impairment Prescribe and administer the loading dose in 2 portions with half of the total dose given as the first portion and the second portion 6 hours later. Write “LOADING Dose” on the prescription Add dose to 50 - 100mL of Sodium chloride 0.9% or glucose 5% Administer using a rate controlled infusion pump over 2 hours Do NOT give as a bolus Oral 500 micrograms PO then a further 500 micrograms 6 hours later loading Write “LOADING DOSE” on the prescription dose Then assess clinically and prescribe maintenance dose if indicated Warning The loading doses may need to be reduced if digoxin or another cardiac glycoside has been given in the preceding two weeks. -

CDER Breakthrough Therapy Designation Approvals Data As of December 31, 2020 Total of 190 Approvals
CDER Breakthrough Therapy Designation Approvals Data as of December 31, 2020 Total of 190 Approvals Submission Application Type and Proprietary Approval Use Number Number Name Established Name Applicant Date Treatment of patients with previously BLA 125486 ORIGINAL-1 GAZYVA OBINUTUZUMAB GENENTECH INC 01-Nov-2013 untreated chronic lymphocytic leukemia in combination with chlorambucil Treatment of patients with mantle cell NDA 205552 ORIGINAL-1 IMBRUVICA IBRUTINIB PHARMACYCLICS LLC 13-Nov-2013 lymphoma (MCL) Treatment of chronic hepatitis C NDA 204671 ORIGINAL-1 SOVALDI SOFOSBUVIR GILEAD SCIENCES INC 06-Dec-2013 infection Treatment of cystic fibrosis patients age VERTEX PHARMACEUTICALS NDA 203188 SUPPLEMENT-4 KALYDECO IVACAFTOR 21-Feb-2014 6 years and older who have mutations INC in the CFTR gene Treatment of previously untreated NOVARTIS patients with chronic lymphocytic BLA 125326 SUPPLEMENT-60 ARZERRA OFATUMUMAB PHARMACEUTICALS 17-Apr-2014 leukemia (CLL) for whom fludarabine- CORPORATION based therapy is considered inappropriate Treatment of patients with anaplastic NOVARTIS lymphoma kinase (ALK)-positive NDA 205755 ORIGINAL-1 ZYKADIA CERITINIB 29-Apr-2014 PHARMACEUTICALS CORP metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Treatment of relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients NDA 206545 ORIGINAL-1 ZYDELIG IDELALISIB GILEAD SCIENCES INC 23-Jul-2014 for whom rituximab alone would be considered appropriate therapy due to other co-morbidities -

Evaluating the Pressor Effects of Drugs & Ambulatory Blood
Characterizing FDA’s Approach to Benefit-Risk Assessment throughout the Medical Product Life Cycle Tommy Douglas Conference Center • Silver Spring, MD May 16th, 2019 9:00 am – 4:55 pm Contents 2 Welcome & Overview Dr. Mark McClellan, MD, PhD, Director, Duke-Margolis Center for Health Policy 9:00 am – 9:05 am Electronic Comments Federal eRulemaking Portal Docket ID: FDA-2019-N-1468 Due June 17, 2019 11:59 PM ET www.regulations.gov/docket?D=FDA-2019-N-1468 Opening Remarks Theresa Mullin, PhD, Associate Director for Strategic Initiatives Center for Drug Evaluation and Research (CDER) 9:05 am – 9:15 am Applying Framework for Benefit-Risk Assessment Throughout the Human Drug Life Cycle Opening Remarks Theresa Mullin, PhD Associate Director Strategic Initiatives FDA Center for Drug Evaluation and Research May 16, 2019 Context • FDA qualitative structured B-R framework – primarily discussed in context of premarket assessment • PDUFA VI (FDARA 2017) enhancing benefit-risk assessment in regulatory decision-making • FDA will further the agency’s implementation of structured benefit-risk assessment, including the incorporation of the patient’s voice in drug development and decision- making, in the human drug review program • FY 2019 --FDA will convene a meeting, conducted through a qualified third party, to gather industry, patient, researcher, and other stakeholder input on key topics. • Including applying the benefit-risk framework throughout the human drug lifecycle, including best approaches to communicating FDA’s benefit-risk assessment • FY -

Guidelines for Use of Digoxin (Lanoxin )
Guidelines for use of Digoxin (Lanoxin) Recommended Neonatal Dose, Route, and Interval Loading or digitalizing dose: Total Loading Dose PMA (Weeks) IV (mcg/kg) PO (mcg/kg) 29 15 20 30 to 36 20 25 37 to 48 30 40 49 40 50 Divide into 3 doses over 24 hours Generally given in the following manner: 1. One-half the loading dose given immediately IV or PO 2. One-fourth the loading dose given 8 to 12 hours later IV or PO 3. The remaining one-fourth loading dose given after an additional 8 to 12 hours IV or PO 4. Administer IV slow push over 5 to 10 minutes 5. Obtain ECG 6 hours after digitalizing dose to assess for toxicity Maintenance dose: should be started 12 hours after the loading dose is completed Maintenance Dose PMA (Weeks) IV (mcg/kg) PO (mcg/kg) Interval (hours) 29 4 5 24 30 to 36 5 6 24 37 to 48 4 5 12 49 5 6 12 Titrate based on clinical response Chief Indications 1. Heart failure caused by diminished myocardial contractility 2. Supraventricular tachycardia, atrial flutter, atrial fibrillation Possible Adverse Reactions 1. Atrial or ventricular arrhythmias are common and may be an early indication of overdosage 2. Feeding intolerance, vomiting, diarrhea 3. Hypokalemia is associated with chronic Digoxin toxicity 4. Bradycardia due to depression of A-V conduction 5. Diuresis from improved cardiac output 6. Lethargy or seizures with toxicity Contraindications & Precautions 1. CAUTION use with pre-existing hypokalemia may lead to adverse reactions 2. CAUTION use with Indomethacin - may inhibit excretion of Digoxin 3.