US5514254.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effects of Metal Ions in Free Radical Reactions Richard Duane Kriens Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1963 Effects of metal ions in free radical reactions Richard Duane Kriens Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Kriens, Richard Duane, "Effects of metal ions in free radical reactions " (1963). Retrospective Theses and Dissertations. 2544. https://lib.dr.iastate.edu/rtd/2544 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. This dissertation has been 64—3880 microfilmed exactly as received KRIENS, Richard Duane, 1932- EFFECTS OF METAL IONS IN FREE RADICAL REACTIONS. Iowa State University of Science and Technology Ph.D„ 1963 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan EFFECTS OF METAL IONS IN FREE RADICAL REACTIONS by Richard Duane Kriens A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Organic Chemistry Approved: Signature was redacted for privacy. In Charge of Major Work Signature was redacted for privacy. ead of Major Departmei^ Signature was redacted for privacy. Iowa State University Of Science and Technology Ames, Iowa 1963 11 TABLE OF CONTENTS Page PART I. REACTIONS OF RADICALS WITH METAL SALTS. ... 1 INTRODUCTION 2 REVIEW OF LITERATURE 3 RESULTS AND DISCUSSION 17 EXPERIMENTAL 54 Chemicals 54 Apparatus and Procedure 66 Reactions of compounds with the 2-cyano-2-propyl radical 66 Reactions of compounds with the phenyl radical 67 Procedure for Sandmeyer type reaction. -

Hydrogen Abstractions from Arylmethanes
AN ABSTRACT OF THE THESIS OF JERRY DEAN UNRUH for the DOCTOR OF PHILOSOPHY (Name) (Degree) in ORGANIC CHEMISTRY presented on ttr:e-( /9 2 O (Major) Title: HYDROGEN ABSTP ArTTnNs FROM ARV! ,TviWTHANES Redacted for Privacy Abstract approved: Dr. 'Gerald Jay Gleicher The relative rates of hydrogen abstraction from a series of 13 arylmethanes by the trichloromethyl radical were determined at 700. The attacking radical was generated from bromotrichloromethane us ing benzoyl peroxide as the initiator.The solvent was benzene- bromotrichloromethane. An excellent correlation, with a coefficient of 0.977, was obtained when the logs of the relative rates of hydrogen abstraction were plotted against the change in pi-binding energy be- tween the incipient radicals and the arylmethanes, if the pi-binding energy were calculated by the SCF approach. When the logs of the kinetic data were correlated with the change in pi-binding energy as calculated by the HMO approach, the correlation was poor, with a co- efficient of 0.855. A possible explanation for this difference might be the HZIckel method's complete neglect of electron interactions. The kinetic data were also plotted against the values of a param- eter,Z1E-3,which are indicative of the ground state electronic environment of the methyl group. A correlation was essentially non- existent.This, coupled with the excellent correlation with the changes in pi-binding energies, indicates that the transition state for hydrogen abstraction by the trichloromethyl radical must certainly lie near the intermediate radical with carbon-hydrogen bond breaking well advanced. The relative rates of hydrogen abstraction from a series of nine arylmethanes by the t-butoxy radical were also determined at 70o. -

Kinetics of Reactions of Bromide and Iodide Ions with Benzyl Chloride and Bromide in Absolute Acetone
DAEHAN HWAHAK HWOEJEE (Journal of the Korean Chemical Society) Vol. 13, Number 2, 1969 Printed in Republic of Korea 벤질 할라이드의 할로겐 교환반응 ( 제 III보 ) 아세톤 중에서의 엳화 및 브롬화 벤질과 브롬화 및 요오드화 이온간의 교환반응 서울대학교 공과대학 응용화학과 황보 명환 • 이 본수 • 이 익춘 (1969. 1. 31. 접수 ) HALOGEN EXCHANGE REACTIONS OF BENZYL HA니 DES Part III- Kinetics of Reactions of Bromide and Iodide Ions with Benzyl Chloride and Bromide in Absolute Acetone. by Myung-Hwan Whangbo, Bon-su Lee and Ikchoon Lee Department of Applied Chemistry, College of Engineering Seoul National University (Received January 31. 1969) ABSTRACT Halogen exchange reactions of benzyl halides have been studied in absolute acetone. Rate constants were calculated using an integrated rate expression derived for the reaction involving ion-pair association. The order of nucleophilicity of halide ions in acetone was found to be a reverse of the order in 90% aqueous enthanol solvent. This was interpreted by means of HSAB principle and solvation of halide ions. Net increase in rate of reaction in acetone compared with the rate in protic solvent resulted from lai■흥 e increase in AS* rather than decrease in △Ht The solvation of the transition state also contribute to the net increase in rate. 요 약 할로겐화 벤질의 할로겐 교환반응을 무수 아세톤에서 연구하였으며 반응속도상수 계산에는 새로 유도 109 — 110 Myung-Hwan Whangbo, Bon-Soo Lee and Ikchoon Lee 한 적분식을 사용하였다 . 아세톤 용매 속에서의 할로겐 이온들의 친핵반응성은 90%에탄올 용매 속에서의 것과 정반대 순서이며 이 것은 HSAB 원리 와 할로겐 이은의 solvation 으로 설명 할 수 있었다 . 아세톤 용매 속에서 의 반응속도의 증가는 ZNF의 감소보다는 z\S手의 증가에 기 인되 며 또 전이 상태의 solvation 현상도 그 원인이 될 수 있음을 논의 하였다 . -

The Thermal Decomposition of Alkyl Benzene Diazo Sulfones
AN ABSTRACT OF THE THESIS OF ROLF STEVEN GABRIELSEN for the Doctor of Philosophy (Name) (Degree) in Chemistry presented on A (Major) (Date) TitleTHE THERMAL DECOMPOSITION OF ALKYL BENZENE- DIAZO SULFONES Redacted for privacy Abstract approved: John L. Kice The thermal decomposition of methyl and benzyl benzenediazo sulfones in various solvents has been studied.In benzene, the de- composition of benzenediazo methyl sulfone (Ph- N =N- SO2CH3) pro- duced biphenyl (0. 32 mole/mole EMS), azobenzene (0. 03 mole/mole 13MS) and sulfur dioxide (0. 14 mole /mole EMS).In cumene, the de- composition led to the formation of considerable benzene and bicumyl, while the decomposition in diphenylmethane produced benzene, benz- hydryl methyl sulfone and tetraphenylethane.These products indicate that decomposition of the methyl diazosulfone in hydrocarbon solvents proceeds in considerable measure by a free radical route.Experi- ments using the Koelsch radical as a radical scavenger in benzene and measurements of the overall rate of decomposition of the diazo- sulfone in the same solvent showed that only about one-third of the molecules of the methyl diazosulfone decomposing yield scavengable free radicals.Possible reasons for this relative low efficiency of radical production are discussed. In contrast to the behavior of the methyl compound, the de- composition of benzenediazo benzyl sulfone (Ph-N=N-S02CH2Ph) in either benzene, cumene or diphenylmethane produced benzaldehyde phenylhydrazone (0. 55-0. 34 mole/mole BBS), N-benzyl benzaldehyde phenylhydrazone -

University Microfilms, Inc., Ann Arbor, Michigan INFLUENCE of SOLVENT
This dissertation has been 62—4152 microfilmed exactly as received HENDRY, Dale Glenn, 1936- INFLUENCE OF SOLVENT ON FREE RADICAL REACTIONS. Iowa State University of Science and Technology Ph.D., 1962 Chemistry, organic University Microfilms, Inc., Ann Arbor, Michigan INFLUENCE OF SOLVENT ON FREE RADICAL REACTIONS by Dale Glenn Hendry A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Organic Chemistry Approved: Signature was redacted for privacy. Signature was redacted for privacy. Signature was redacted for privacy. Dean f Graduate College Iowa State University Of Science and Technology Ames, Iowa 1962 ii TABLE OF CONTENTS Page GENERAL INTRODUCTION ' 1 PHDTOCHLQRIMTION OF ARALKYL HYDROCARBONS 5 Introduction 5 Results and Discussion 11 Relative reactivity of alpha- and beta-hydrogen atoms 11 Reactivity of alpha- and beta-hydrogen atoms relative to cyclohexane 17 Experimental 33 ^paratus and procedure 33 Analysis procedure 3li Reagents 35 METHYLATION OF HYDROCARBONS 36 Introduction 36 Results and Discussion 39 Experimental Ui Apparatus and procedures for methylation reactions UU Analys is k5 Reagents U6 OXIDATION OF HYDROCARBONS kl Introduction 1+7 Results and Discussion $1 Oxidation kinetics of cyclohexene $1 Study of rate of initiation in various solvents 55 Solvent effect on oxidation of hydrocarbons 61 Relative reactivity of cyclic hydrocarbons toward the peroxy radical 8l Experimental 86 Oxidation procedure 86 Reagents 8? iii Page OXIDATION OF HYDROCARBONS IN THE PRESENCE OF TRIPHENYLMETHANE AND SIMILAR TYPE COMPOUNDS 89 Introduction 89 Results and Discussion 91 Experimental 119 Procedure 119 Reagents 119 SUMMARY 120 REFERENCES CITED 122 ACKNOWLEDGMENTS 130 XV LIST OF TABLES Table Page 1 Photochlor inat i on of aralkyl hydrocarbons at U0° 12 2 Competitive photochlorination of cyclohexane and aralkyl hydrocarbons at U0° 18 3 Reactivities of some carbon-hydrogen bonds toward chlorine atoms at U0° 22 U Relative reactivities of some aralkyl hydrocarbons . -

Heavy-Atom Kinetic Isotope Effects
A UNITED STATES DEPARTMENT OF COMMERCE NATL PUBLICATION NBS SPECIAL PUBLICATION 349 •Nr., of** Heavy-Atom Kinetic Isotope Effects An Indexed Bibliography U.S. EPARTMENT OF COMMERCE National Bureau of jQ^^^tandards 00 SI — NATIONAL BUREAU OF STANDARDS The National Bureau of Standards^ was established by an act of Congress March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measure- ment system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to promote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Center for Computer Sciences and Technology, and the Office for Information Programs. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scien- tific community, industry, and commerce. The Institute consists of a Center for Radia- tion Research, an Office of Measurement Services and the following divisions: Applied Mathematics—Electricity—Heat—Mechanics—Optical Physics—^Linac -

Locating and Estimating Sources of Toluene
LOCATING AND ESTIMATING AIR EMISSIONS FROM SOURCES OF TOLUENE Final Report Prepared for: Dennis Beauregard Emission Inventory Branch Technical Support Division Office of Air Quality Planning and Standards U.S. Environmental Protection Agency Research Triangle Park, North Carolina 27711 Prepared by: TRC Environmental Corporation 100 Europa Drive, Suite 150 Chapel Hill, North Carolina 27514 September 1993 EPA Contract No. 68-D9-0173 Work Assignment No. 3/316 DISCLAIMER This report was furnished to the U.S. Environmental Protection Agency by TRC Environmental Corporation, 100 Europa Drive, Chapel Hill, North Carolina, 27514, in partial fulfillment of Contract No. 68-D9-0173, Work Assignment No. 3/316. This document has been reviewed by the Office of Air Quality Planning and Standards and has been approved for publication. The opinions, findings, and conclusions expressed are those of the authors and not necessarily those of the U.S. Environmental Protection Agency. Mention of company or product name is not to be considered as an endorsement by the U.S. Environmental Protection Agency. ii TABLE OF CONTENTS Section Page DISCLAIMER ..................................................... ii LIST OF FIGURES .................................................. vi LIST OF TABLES .................................................. viii 1.0 PURPOSE OF DOCUMENT ...................................... 1-1 1.1 Reference for Section 1.0 ..................................... 1-5 2.0 OVERVIEW OF DOCUMENT CONTENTS ........................... 2-1 2.1 References for Section 2.0 .................................... 2-5 3.0 BACKGROUND ............................................... 3-1 3.1 Nature of Pollutant ......................................... 3-1 3.2 Overview of Production and Use ................................ 3-4 3.3 References for Section 3.0 .................................... 3-7 4.0 EMISSIONS FROM TOLUENE PRODUCTION ........................ 4-1 4.1 Toluene Production from Petroleum Fractions ...................... -

The University of New South Wales School of Chemical
THE UNIVERSITY OF NEW SOUTH WALES SCHOOL OF CHEMICAL ENGINEERING DIFFUSION AND KINETIC STUDIES IN THE CHLORINATION OF TOLUENE DISSERTATION Submitted in Partial Fulfilment of the Requirement for the Degree of Doctor of Philosophy in Chemical Engineering FONG SECK KONG B. Sc. Hons. I January 1970. 0F KE,^30/^' (, -x- KENSINGTON ) V * . <fJ Libram CANDIDATE'S CERTIFICATE This is to certify that the work presented in this thesis was carried out in the School of Chemical Engineering of the University of New South Wales, and has not been submitted to any other University or Institution for a Higher Degree. (Fong Seek Kong). 1 Acknowledgements The author wishes to record his sincere thanks to Professor J.S. Ratcliffe who suggested and supervised the work described in this thesis. His constant interest and discussions concerning the work have been an inspiration. The author is further thankful to Professor J.S. Ratcliffe and to Miss Chamberlain of the Commonwealth Office of Education and Science for their patient discussions and kind advice in difficult times. Thanks are due to the Commonwealth Government of Australia for the scholarship under the Commonwealth Scholarship and Fellowship Plan, and to the Government of Singapore for securing the award. Thanks are also due to the professional and technical staff of the School of Chemical Engineering for their assistance in the equipment construction and advice rendered, and to Mrs. V. Theodore for typing this thesis. Last, but not least, the author is greatly indebted to his family, whose days of austerity have to be prolonged, and to Miss Chew Ann Kheng for their encouragement and support. -

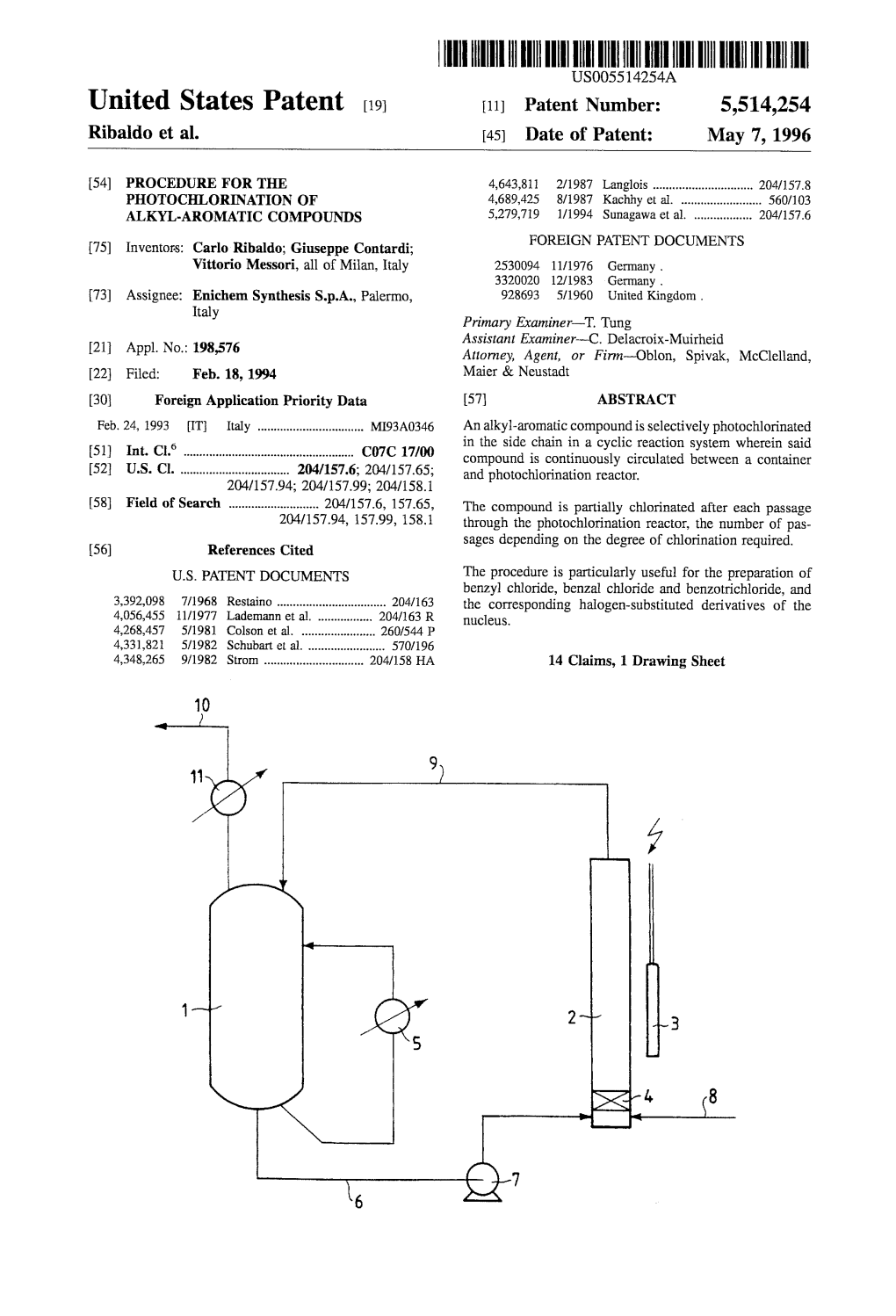
Procedure for the Photochlorination of Alkyl-Aromatic Compounds
~™ llll III II II II I II I II III II 1 1 (19) J European Patent Office Office europeen des brevets (11) EP 0 612 707 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int. CI.6: C07C 17/14, C07C 22/04, of the grant of the patent: C07C 25/02 18.06.1997 Bulletin 1997/25 (21) Application number: 94200350.0 (22) Date of filing: 16.02.1994 (54) Procedure for the photochlorination of alkyl-aromatic compounds Verfahren zur Photochlorierung von alkylaromatischen Verbindungen Procede pour la photochloration de composes alkylaromatiques (84) Designated Contracting States: • Contardi, Giuseppe AT BE CH DE DK ES FR GB GR IE IT LI LU MC NL 1-20161 Milan (IT) PTSE • Messori, Vittorio 1-20129 Milan (IT) (30) Priority: 24.02.1993 IT MI930346 (74) Representative: Fusina, Gerolamo et al (43) Date of publication of application: Ing. Barzanb & Zanardo Milano S.p.A, 31.08.1994 Bulletin 1994/35 Via Borgonuovo, 10 20121 Milano (IT) (73) Proprietor: ENICHEM SYNTHESIS S.p.A. 1-90139 Palermo (IT) (56) References cited: DE-A- 3 320 020 DE-B- 2 530 094 (72) Inventors: GB-A- 928 693 • Ribaldo, Carlo 1-20131 Milan (IT) CO o Csl Note: Within nine months from the publication of the mention of the grant of the European patent, give CO any person may notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall be filed in ^ a written reasoned statement. It shall not be deemed to have been filed until the opposition fee has been paid. -

Synthetic and Mechanistic Studies of Poly(Vinyl Chloride) and Some Other Chlorinated Polymers
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 2003 Synthetic and mechanistic studies of poly(vinyl chloride) and some other chlorinated polymers Xianlong Ge College of William & Mary - Arts & Sciences Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Organic Chemistry Commons, and the Polymer Chemistry Commons Recommended Citation Ge, Xianlong, "Synthetic and mechanistic studies of poly(vinyl chloride) and some other chlorinated polymers" (2003). Dissertations, Theses, and Masters Projects. Paper 1539623417. https://dx.doi.org/doi:10.21220/s2-drc1-7f95 This Dissertation is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. SYNTHETIC AND MECHANISTIC STUDIES OF POLY(VINYL CHLORIDE) AND SOME OTHER CHLORINATED POLYMERS A Dissertation Presented to The Faculty of the Department of Applied Science The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Doctor of Philosophy by Xianlong Ge 2003 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. APPROVAL SHEET This dissertation is submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Xianlong Ge Approved, May 2003 llliam H. Starnes, Jr. Robert A. Orwoll Robert L. Void Robert D. Pike Department of Chemistry Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. TABLE OF CONTENTS ACKNOWLEDGMENTS..............................................................................................................viii LIST OF TABLES.................................................................................................................. -

Photochlorination of Aliphatic Hydrocarbons Philip Gene Haffley Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1967 Photochlorination of aliphatic hydrocarbons Philip Gene Haffley Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Haffley, Philip Gene, "Photochlorination of aliphatic hydrocarbons " (1967). Retrospective Theses and Dissertations. 3393. https://lib.dr.iastate.edu/rtd/3393 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. PHOTOCHLORINATION OF ALIPHilTIC HYDHOCAHBOÎIS "by Philip C-ene Haffley A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Eequirments for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Organic Chemistry At>riroved: Signature was redacted for privacy. In Charge of Major Work Signature was redacted for privacy. Signature was redacted for privacy. Iowa State university Of Science and Technology Ames, Iowa 1967 ii TABLE OP CONTENTS Page LITSPJl'rUHE SURVEY 1 INTRODUCTION 11 RESULTS MvD DISCUSSION l4 Photochlorination of Methylpentanes 14 Chlorination of 2,^-Dimethylhezane 23 Effect of Solvent on the Photochlorination of 43 Compoimds Containing a Polar Group Photochlorination -

Polar Effects in Free Radical Reactions Roger Clinton Williamson Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1963 Polar effects in free radical reactions Roger Clinton Williamson Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Williamson, Roger Clinton, "Polar effects in free radical reactions " (1963). Retrospective Theses and Dissertations. 2952. https://lib.dr.iastate.edu/rtd/2952 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. POLAE EFFECTS IN FREE BADICAL REACTIONS by Roger Clinton Williamson, Jr. A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject! Organic Chemistry Approved: Signature was redacted for privacy. In Charge of Major Work Signature was redacted for privacy. Signature was redacted for privacy. lajh. of Graduate Colle, Iowa State University Of Science and Technology Ames, Iowa 1963 ii TABLE OP CONTENTS Page GENERAL INTRODUCTION 1 PHOTOCHLOEINATION OF SUBSTITUTED TOLUENES AND AUTOXIDATION OF SUBSTITUTED CUMENES, STYRENES AND BENZYL PHENYL ETHERS 12 Introduction 12 Results and Discussion 28 Experimental 70 Photochlorination